Glass Vacuum Trap Safety Alert
Incident Overview
On the evening of Saturday, October 9th, 2021, an over-pressurized glass vacuum trap exploded on a high vacuum line in a Chemistry laboratory.
After careful investigation, we believe that rapidly expanding oxygen gas from condensed liquid oxygen in the sealed trap caused the trap to explode after the liquid nitrogen dewar was removed while shutting down the trap after use. No volatile organics had been co-condensed with the oxygen.
The explosion caused lacerations and major bleeding to the face of a scientist standing nearby. The Evanston Fire Department transported the scientist to the emergency room for care, and they were released hours later. Fortunately, the proper use of Personal Protective Equipment (PPE) and a prompt call for help mitigated the severity of the injuries.
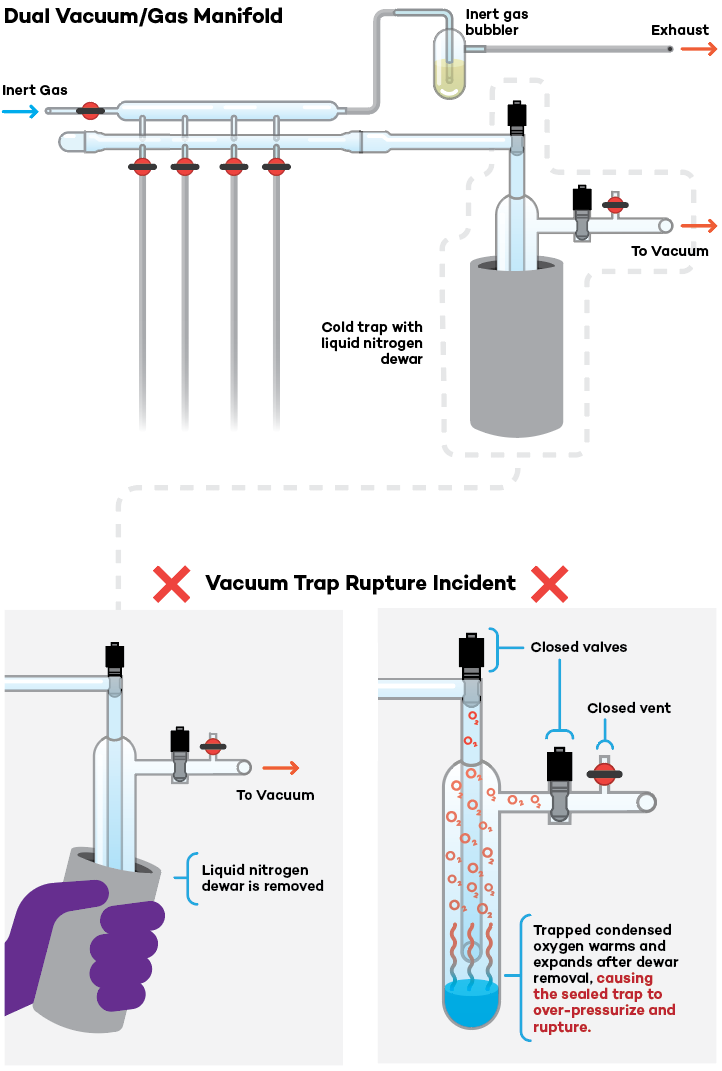
Background
Vacuum traps cooled with liquid nitrogen can condense common gases and require extreme caution during use.
- Condensed liquid oxygen may accumulate if ambient air enters the trap.
- Condensed liquid oxygen is often evident by a characteristic blue hue. Note: the blue color may NOT be readily apparent if ice/frost accumulates on the glass trap surface or if there are co-condensed volatiles.
- Air can access a vacuum line through leaks in the system or improper shutdown procedures. Liquid oxygen can violently react with readily-oxidized substances in the trap, such as organic solvents and vacuum grease. Furthermore, liquid oxygen poses an over-pressurization hazard if the trap is sealed or improperly vented during shutdown.
- Other gases either generated by a reaction or leaking into the vacuum line can also condense in the trap. Valves meant to deliver gases such as argon to a chamber, vessel, or line under active vacuum may be a source of condensable gas if those valves leak, are not seated properly, are old/damaged, or are not entirely closed.
Best Practices and Lessons Learned
If you are working with cold vacuum traps, then please do the following:
- Maintain up-to-date standard operating procedures on vacuum line and cold trap operation in your lab.
- Regularly train all operators on proper use.
- Never work alone with hazardous materials or physical hazards in the lab.
- Regularly test the integrity of your vacuum system and apparatus for potential leaks. Poor vacuum readings observed via an installed vacuum gauge may indicate a possible leak.
- Use trap cooling sources that do not condense common gases—such as a dry ice/solvent mixture—when possible. Use liquid nitrogen to cool a trap only when absolutely necessary.
- Always wear correct PPE (lab coat, cryogen gloves, splash goggles, face shield) when dispensing and working with liquid nitrogen, and always work with liquid nitrogen in well-ventilated areas.
- Operate cold vacuum traps inside a certified laboratory fume hood. Use the fume hood sash as a physical barrier between the user and the system at all times.
- If working inside a laboratory fume hood is impossible, use a blast shield and wear a face shield with splash goggles when removing the dewar.
- When removing the cold source from the trap, be sure to inform everyone in the immediate area.
- Always vent the trap immediately after removing the liquid nitrogen source and turning the vacuum pump off; leaving the trap completely sealed beyond this point could result in sudden over-pressurization if condensed gas is present.
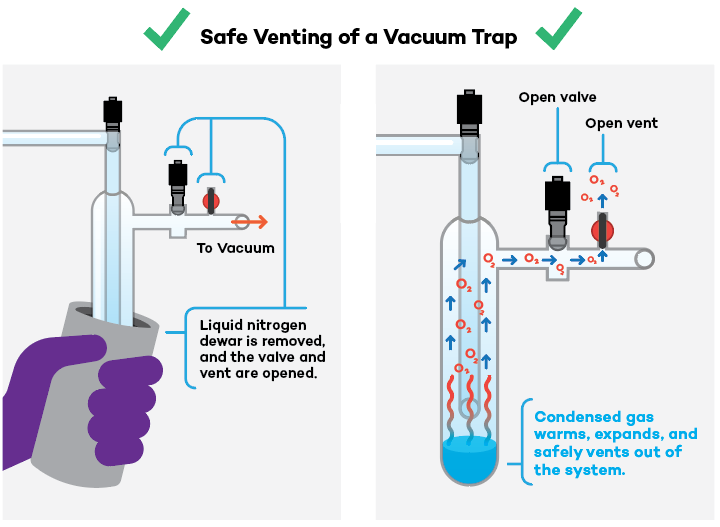
- Conversely, never vent the trap while it is immersed in liquid nitrogen, which could also introduce condensable oxygen into the system.
- If there are any questions, Research Safety can help.
Liquid oxygen poses unique and significant safety concerns when co-condensed with organic material. If you observe or suspect that oxygen has condensed with readily-oxidized volatiles in your liquid nitrogen vacuum trap as you remove the dewar:
- Immediately return the dewar back onto the trap to maintain liquid nitrogen contact with the trap;
- Close the fume hood sash completely;
- Notify others and evacuate the lab;
- Immediately contact Research Safety (during normal business hours) or University Police at 456 (outside business hours).
Research Safety Contact
Evanston Campus
Technological Institute, 2145 Sheridan Road, Room NG-71
Phone (847) 491-5581
Chicago Campus
345 East Superior Street, Suite 1522
Phone (312) 503-8300