IBC Registration and Review Process
How it Works
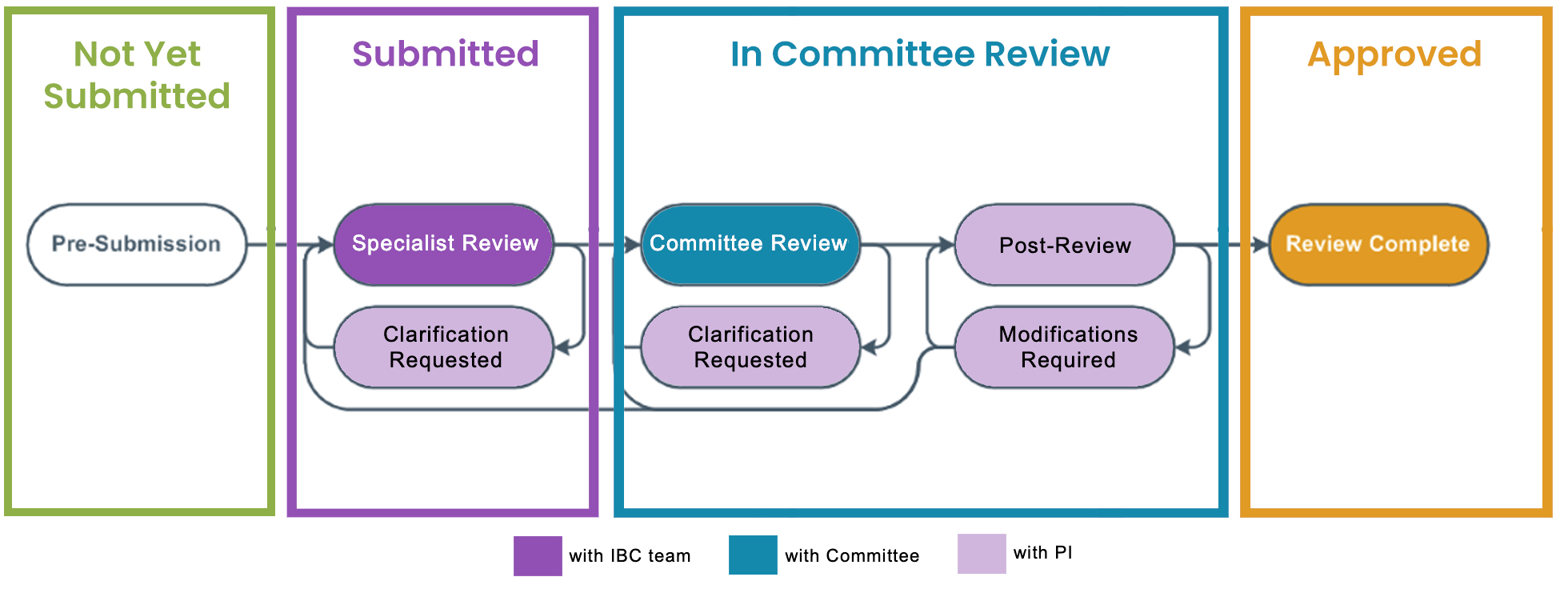
- During the Pre-Submission state the lab answers the form questions listed in eIBC.
- Once the lab has completed the Biological registration questionnaire, the lab submits the registration for pre-review by our IBC team under Specialist Review.
- The IBC team will make recommendations for clarification.
- The PI/PI Proxy revises the registration and then submits it to the IBC team for Committee review. Committee members have 5 business days to complete the initial review.
- The registration is returned to the PI/PI Proxy to make any requested changes.
- The registration is submitted back for the second round of committee review. Once all issues are resolved, the registration is moved for discussion during the next committee meeting. IBC meetings are held on the 3rd Wednesday of each month.
- At the monthly IBC meeting all committee members review, discuss, and make a determination for each registration. All determinations are sent out within 24 hours of the meeting. Biological Registration in the Approved state are in compliance with Federal, State, and local regulations.
Types of Submissions
Initial
Initial submissions are ___
De Novo
De Novo submissions are ___
Amendment
Amendments are ______
Amendment/CR
Amendment/CR are __________
Continuing Review
Continuing Reviews are __________
