- Home
- Safety Information
- Laboratory Safety and Chemical Hygiene Plan
Laboratory Safety and Chemical Hygiene Plan
Last Updated: March 20231.0 Introduction
A sustainable safety culture in research is built on leadership engagement, hazard awareness, enhanced communication, and behavior changes. The Laboratory Safety and Chemical Hygiene Plan provides information and guidance to help conduct laboratory work safely and in compliance with environmental health and safety regulations and University policy. It is also a useful training resource for principal investigators, other supervisory personnel, safety designates, and laboratory members. While it primarily addresses work in laboratory spaces, much of the information is applicable to non-laboratory spaces where hazardous chemicals or processes are used.
The Laboratory Safety and Chemical Hygiene Plan is required by the Occupational Safety and Health Administration (OSHA) Laboratory Standard 29 CFR 1910. 1450. The Lumen Profile is the laboratory-specific portion of the chemical hygiene plan. Lumen Profiles are required for all research, research-support, and teaching laboratory spaces.
Although the information in this document is compiled from sources believed to be reliable, it is not all-encompassing and is intended only to serve as a starting point for good laboratory practice. The laboratory manager or supervisor is responsible for laboratory-specific information, for developing and maintaining a safe workplace, and for complying with federal, state, and local laws and University policy.
Whenever used, the word shall indicates required procedures. The word should indicates a recommendation for good practice.
Policies and procedures for work with radioactive materials are administered by the Radiation Safety Committee in the Radiation Safety Handbook. The Laser Safety Handbook covers policies and procedures for work with lasers. Policies and procedures for work with potentially infectious agents and recombinant DNA are covered in the Institutional Biological Safety Manual, which references the CDC Biosafety in Microbiological and Biomedical Laboratories, 5th Ed. and the OSHA Bloodborne Pathogen Program.
This document has been reviewed by the Laboratory and Chemical Safety Committee and approved by the Vice President for Research.
1.1 Federal Laws and Regulations
There are a number of federal, state, and local laws, regulations, ordinances and standards that pertain to laboratory activities and conditions that affect the environment, health and safety. International regulations apply to air and marine transport of laboratory materials. Safety standards and codes are created by nongovernmental bodies, but are important to know because they may be required by a law (by reference), as condition of occupancy, by an insurance company, by an accrediting body, or as a p. 7 widely accepted industry standard. In some cases, following a safety guideline is a condition of receiving a research grant1.
There are a number of guideline setting governmental and non-governmental agencies. The main guideline for chemical and laboratory safety is the National Research Council’s Prudent Practices in the Laboratory 2011, which is referenced throughout this document.
Other notable agencies include the National Fire Protection Agency (NFPA), the National Institute for Occupational Safety and Health (NIOSH), National Institutes of Health (NIH), the National Toxicology Program (NTP), the Compressed Gas Association (CGA), the American Conference of Governmental Industrial Hygienists (ACGIH), the American Chemical Society (ACS), the American Industrial Hygiene Association (AIHA) , the American Institute of Chemical Engineers (AIChE) and other standard developing agencies under the American National Standards Institute (ANSI).
Table 1.1
Federal Safety Laws and Regulations That Pertain to Laboratories – Regulations of Chemical, Physical and Mechanical Hazards in Laboratories2.
| LAW OR REGULATION | CITATION | PURPOSE | COMMENTS |
|---|---|---|---|
| Occupational Safety and Health Act (OSHA) | 29 USC § 651 et seq. | Worker protection | |
| General Duty Clause | 29 USC § 654(5)(a) and (b) | Assurance of workplace free from recognized hazards that are causing or likely to causeserious physical harm | Foundation enforceable requirement in absence of a specific standard |
| Occupational Exposure to Hazardous Chemicals in Laboratories (Laboratory Standard) | 29 CFR § 1910.1450 | Laboratory worker protection from exposure to hazardous chemicals | Requires a chemical hygiene plan. Title 29 rules are written and enforced by OSHA. |
| Hazard Communication Standard | 29 CFR § 1910.1200 | General worker protection from chemical use | Requires training of employees, provision of Safety Data Sheets (SDS) by manufacturer, labeling of pipes and containers containing hazardous materials and list of hazardous chemicals. |
| Air Contaminants | 29 CFR §§ 1910.1000–1910.1050 | Standards for exposure to hazardous chemicals | Chemical-specific regulations pertinent in laboratories |
| Hazardous Materials | 29 CFR §§ 1910.101–1910.111 | Protection against hazards of compressed gases, flammable and combustible liquids, explosives, anhydrous ammonia | See also Uniform Fire Code and National Fire Protection Association standards |
| OSHA Respiratory Protection Standard | 29 CFR § 1910.134 | When respiratory protection is required; how to fit and use respirators; and medical review | Requires risk assessment and implementation of hierarchy of controls such as substitution, engineering and administrative controls prior to PPE implementation. |
| Personal Protective Equipment | 29 CFR §§ 1910.132–1910.138 | Head, hand, foot, eye, face, and respiratory tract protection | See also American National Standards Institute standards |
| Control of Hazardous Energy (Lock out/Tag out) | 29 CFR § 1910.147 | Worker protection from electrical and other stored energy hazards | |
| Machinery and Machine Guarding | 29 CFR §§ 1910.211–1910.219 | Worker protection from mechanical hazards | |
| Controlled Substances | 21 CFR §§ 1300-1399 | Requires licenses and controls for the purchase, use, and possession of controlled substances, illicit drugs, and certain drug precursors | Enforced by the Drug Enforcement Agency |
| Chemical Facility AntiTerrorism Standards (CFATS) with Appendix | 6 CFR Part 27 | Establishes risk-based performance standards for the security of chemical facilities | Northwestern researchers inventory a subset of Appendix A applicable to laboratories |
| Toxic Substances Control Act (TSCA) Polychlorinated biphenyls (PCBs) | 40 CFR Part 761 | Prohibition against PCBs in manufacturing, processing, distribution in commerce, and certain uses | Permits certain limited laboratory use of PCBs |
| Permit and Excise Tax for Purchase of 190 and 200-proof Ethanol | 27 CFR Part 211 | Control of the sale of ethanol | Enforced by the U.S. Bureau of Alcohol, Tobacco, and Firearms |
Table 1.2
Federal Safety Laws and Regulations That Pertain to Laboratories – Environmental Regulations
| LAW OR REGULATION | CITATION | PURPOSE | COMMENTS |
|---|---|---|---|
| Resource Conservation and Recovery Act (RCRA) | 42 USC § 6901 et seq. | Protection of human health and environment | |
| Hazardous Waste Management | 40 CFR Parts 260–272 | “Cradle-to-grave” control of chemical waste from laboratories. Subpart K applies. | Subpart K of 40 CFR Part 262 is an opt-in rule specific to laboratories in academia. Title 40 rules are written and enforced by EPA |
| Clean Air Act (CAA) | 42 USC § 7401 et seq. | Protection of air quality and human health | |
| CAA Amendments of 1990 | 42 USC § 7409 et seq. | Expansion of air quality protection | Requires development of specific rules for laboratories |
| National Emission Standards for Hazardous Air Pollutants | 40 CFR Part 82 | Control of air pollutant emissions | |
| Montreal Protocol for Protection of Stratospheric Ozone | 40 CFR Part 82 | Control of emission of ozonedepleting compounds | Severely limits use of certain chlorofluorocarbons |
| Federal Water Pollution Control Act | 33 USC § 1251 et seq. | Improvement and protection of water quality | |
| Criteria and standards for the National Pollutant Discharge Elimination System (NPDES) | 40 CFR Part 125 | Control of discharge to public waters | |
| General Pretreatment Regulations for Existing and New Sources of Pollution | 40 CFR Part 403 | Control of discharge of pollutants to public treatment works | Implemented by local sewer authorities |
Table 1.3
Federal Safety Laws and Regulations That Pertain to Laboratories – Shipping, Export, and Import of Laboratory Materials
| LAW OR REGULATION | CITATION | PURPOSE | COMMENTS |
|---|---|---|---|
| Hazardous Materials Transportation Act | 48 USC § 1801 et seq. | Control of movement of hazardous materials | |
| Hazardous Material Regulations | 49 CFR Parts 100–199 | Regulation of packaging, labeling, placarding, and transporting | Standards of the International Air Transport Agency apply to chemicals shipped by air. Title 49 rules are written and enforced by DOT. |
| Hazardous Materials Training Requirements | 49 CFR §§ 172.700–172.704 | Assurance of training for all persons involved in transportation of hazardous materials | Also known as HM126F |
| TSCA | 15 USC § 2601 et seq. | Requires testing and necessary restrictions on use of certain chemical substances | Collection and development of information on chemicals in commerce |
| Reporting and Recordkeeping Requirements | 40 CFR Part 704 | One provision exempts users of small quantities solely for research and development R&D) | Must follow R&D exemption requirements |
| Significant Adverse Reaction | 40 CFR Part 717 | Record of new allegation that chemical substances or mixture caused significant adverse effect for health or the environment | TSCA § 8(c) |
| Technically Qualified Individual (TQI) | 40 CFR § 720.3(ee) | Definition of TQI by background; understanding of risks, responsibilities, and legal requirements | Follow TQI requirements with R&D |
| TSCA Exemption for R&D | 440 CFR § 720.36 | Exemption for R&D from PMN if chemical substance not on TSCA inventory or is manufactured or imported only in small quantities solely for R&D | Follow R&D exemption requirements including labeling and SDS information |
| Exports of Samples, Chemicals, Biologicals, Other Materials, and Laboratory Equipment | 15 CFR Parts 730–774 | Regulates shipments of certain chemicals and other research materials out of the United States | These rules are administered by the U.S. Department of Commerce; other export regulations may apply |
Table 1.4
Federal Safety Laws and Regulations That Pertain to Laboratories – Regulation of Laboratory Injuries, Accidents, and Spills
| LAW OR REGULATION | CITATION | PURPOSE | COMMENTS |
|---|---|---|---|
| Public Health Security and Bioterrorism Preparedness and Response Act of 2002 | 42 CFR Part 73 | Establishes the requirements for possession, use, and transfer of select agents and toxins | Select agents are biological agents that are a terror risk. Rules are administered by the U.S. Centers for Disease Control and Prevention and the U.S. Animal and Plant Health Inspection Service |
| Recording and Reporting Occupational Injuries and Illnesses | 29 CFR Part 1904 | Standards for employee reporting and recordkeeping | |
| Employee Emergency Plans and Fire Prevention Plans | 29 CFR § 1910.38 | Requirements for written emergency and fire prevention plans | |
| Medical Services and First Aid | 29 CFR § 1910.151 | Provision of medical services, first-aid equipment, and facilities for quick drenching and flushing of eyes | |
| Superfund Amendments and Reauthorization Act (SARA) |
42 USC § 9601 et seq 42 USC § 11000 et seq. 40 CFR Part 370 (§ 311–312) 40 CFR Part 372 (§ 313) |
Planning for emergencies andreporting of hazardous materials | Title III, also known as Community Right-to-Know Act |
| Emergency Planning and Notification | 40 CFR Part 355 | Requirements for reporting of extremely hazardous materials and unplanned releases | Applies to all chemical users |
| Hazardous Waste Operations and Emergency Response |
29 CFR § 1910.120 40 CFR Part 311 |
Worker protection during hazardous waste cleanup | Applies to state and local government employees not covered by OSHA |
Table 1.5
Federal Safety Laws and Regulations That Pertain to Laboratories – Other regulations
| LAW OR REGULATION | CITATION | PURPOSE | COMMENTS |
|---|---|---|---|
| Americans with Disabilities Act | 28 CFR Part 36 | Standards for making workplace accommodations for students and employees with disabilities | |
| Access to Employee Exposure and Medical Records | 29 CFR § 1910.20 | Employee and privacy and other rights; employer responsibilities | |
| Occupational Noise Exposure | 29 CFR § 1910.95 | Standards for noise, monitoring and medical surveillance |
1.2 Local Laws
- City of Evanston Recovery of Hazardous Substance Removal and Abatement Costs Ordinance: provides for recovery of emergency response costs.
- City of Chicago Liability for Fire Suppression and Other Emergency Costs Ordinance: provides for recovery of emergency response costs.
- The Metropolitan Water Reclamation District of Greater Chicago Sewage and Waste Control Ordinance.
1.3 Regulation of Laboratory Design and Construction
Laboratory design, construction, and renovation are regulated mainly by state and local laws that incorporate, by reference, generally accepted standard practices, such as the International Building Code (IBC) 2012, the International Fire Code (IFC) 2012 in Evanston, and the National Fire Protection Association (NFPA) standards and the City of Chicago Building Code in Chicago. For laboratory buildings where hazardous chemicals are stored or used, detailed requirements usually cover spill control, drainage, containment, ventilation, emergency power, special controls for hazardous gases, fire prevention, building height, and allowable quantities.
In addition, OSHA standards affect some key laboratory design and construction issues, for example eyewashes, safety showers, and special ventilation requirements. Other consensus standards prepared by organizations such as the American Society of Heating, Refrigeration, and Air Conditioning Engineers (ASHRAE) are relevant to laboratory space design. It is common for various codes and consensus standards to be incorporated into state or federal regulations.
2.0 Responsibilities
This section describes and assigns those responsibilities that deal directly with laboratories using chemicals, biological materials, radioactive materials, other sources of ionizing radiation, and/or lasers.
All faculty, staff, and students are required to complete mandatory training assigned through Lumen based on their hazard and risk profile. For some training, this is an annual requirement. For other training, it only needs to be completed once.
2.1 Vice President for Research
The President of the University has delegated administrative responsibility for the chemical, biological, radiation, and laser safety programs to the Vice President for Research.
2.2 Department Heads, Center Directors, and Other Facility Directors
The term department head will be used in this text to include center directors and other facility directors.
Department heads should coordinate with Northwestern Environmental Health and Safety to develop evacuation plans for buildings, appoint building safety committees, and appoint building safety managers and alternates.
The department head shall maintain discipline, enforce rules and regulations, and take prompt, effective corrective action when necessary. The department head shall also provide assistance to Research Safety staff when situations arise involving PIs and other personnel in the department.
The department head shall be familiar with and understand the federal, state, and local regulations and University policies applicable to the department’s work and shall ensure compliance through PIs and other supervisory personnel.
The department head may delegate safety and health-related responsibilities to PIs or other supervisors, but it is the department head’s responsibility to see that the requirements are met.
The department head is responsible for ensuring that engineering controls and safety equipment are inspected and maintained according to the required maintenance schedule. The department head shall ensure that the PIs institute medical surveillance programs for personnel with occupational exposure to certain agents.
When a PI vacates a laboratory space, the department head is responsible for ensuring that the lab space is properly cleaned out and prepared for the next occupant. The department head shall be responsible for providing decontamination of the lab space if the PI fails to do so.
2.3 Principal Investigators and Shared and Core Facilities Managers
The term principal investigator (PI) as used in this document shall include laboratory and other supervisors (such as managers or directors of shared and core facilities). A PI is defined as any faculty member who has been granted permission by the Office for Sponsored Research to serve as a PI on a project or to submit a proposal. All persons granted faculty-level research appointments are eligible to be PIs. The Vice President for Research may authorize others to be PIs or core facility managers for the Office for Research.
The PI is responsible to the department head for the safe and legal conduct of research under his or her purview. Shared or core facility managers are responsible to the faculty director and the respective administration. This safety responsibility shall not be delegated. The PI shall be aware of the physical and health hazards associated with all materials present in the laboratory space. The PI shall record all acquisitions of Chemicals of Interest within 30 days.
The supervisor of a teaching laboratory is also considered a PI in the context of the Research Management Platform Lumen. The supervisor of a teaching laboratory does not need to register students in Lumen who participate in laboratory activities as part of a Northwestern University class. Laboratory teaching assistants are required to be registered as laboratory workers in Lumen.
In the event of an incident including infectious exposure, the PI shall initiate appropriate emergency procedures, follow the incident reporting requirements and formulate a temporary laboratory shutdown plan.
The PI shall add all supervised laboratory members to their Lumen Profile, make all laboratory members aware of the profile, and enforce the safety rules and procedures described therein. The PI shall be familiar with and understand the rules, regulations, and University policies pertaining to the workplace. These encompass but are not limited to the following items: standard operating procedures, training, record keeping, maintaining and providing easy access to SDSs, labeling chemicals, labeling and proper disposal of surplus and waste materials, posting warnings, medical surveillance, inventory reporting, engineering controls, safe work practices, personal protective clothing and equipment, and access restrictions.
The Occupational Safety and Health Administration (OSHA) states that it is “clear that it is the employer’s responsibility to compel compliance… it is the employer, and not the employee, who controls the conditions of work at a given workplace.” According to University interpretation, the PI is defined as the individual responsible for ensuring adherence to safety regulations and proper use of safety equipment in the laboratory space. The PI shall correct any deficiencies that could compromise health and safety or compliance.
The PI shall not assign pregnant women to clean hazardous materials spills or work with chemicals that are particularly hazardous to a fetus.
When closure of a laboratory space becomes imminent, the PI is responsible for following the lab closeout process in the Laboratory Closeout Checklist. The PI ensures removal of all chemical and other health and safety hazards so the laboratory space is safe for renovation or subsequent use. The PI shall report to the department head that all hazardous materials have been removed and work surfaces (lab furniture, refrigerators, freezers, chemical fume hoods, etc.) decontaminated with an appropriate and Research Safety-approved method. Should a PI abandon hazardous material, the department becomes responsible for arranging disposal, both in terms of inventory and funding.
The PI may choose a proxy or Safety Designate to enter information into the Lumen Profile; however, it is the PI/Supervisor responsibility to verify that the data is complete and accurate.
The Safety Designate shall be a senior lab member. It is inappropriate to delegate the Safety Designate role to a junior member of the lab team.
2.4 Laboratory Members
Each individual who works in a laboratory where hazardous materials are used is considered a laboratory member. Each laboratory member shall know and comply with the University’s safety policies and rules and shall follow both oral and written instructions from the PI or supervisor. The individual shall report to the PI any unsafe conditions and any accident or exposure. If the individual receives no response or an unsatisfactory response, (s)he shall contact the department head or Research Safety. The department head or Research Safety shall ensure confidentiality for the individual reporting a safety concern. Northwestern University has selected EthicsPoint to provide a simple way to report activities that may involve misconduct or violations of Northwestern University policy. Laboratory safety concerns may also be submitted directly to Research Safety using the ObservNow function in Lumen.
The individual shall know the hazards in the workplace as well as proper hazardous material handling and disposal procedures. Training shall be provided or arranged by the PI. Avoid working alone in a building; do not work alone in a laboratory if the procedures being conducted are hazardous3.
Research Safety encourages pregnant women to voluntarily declare their pregnancy and receive consultation from an occupational health services provider. Contact Risk Management for a free Clinic Passport and bring a list of hazardous chemicals, infectious materials or radioactive materials you are exposed to in your occupational environment.
If you are pregnant or planning to become pregnant, contact Research Safety so we can provide guidance in minimizing an exposure risk.
2.5 Students
Although the federal and state laws apply only to employees (including student employees), it is the policy of Northwestern University to ensure that all students who might be exposed to hazardous materials in the course of their activities at the University are also adequately protected and trained. Therefore, Lumen Profiles shall also be prepared for teaching laboratories. The laboratory supervisor shall instruct students in the appropriate safety precautions and enforce the given rules.
Working alone is especially dangerous when those involved have little research experience, such as undergraduates or those who have not demonstrated competency working with a new hazard. Working alone in a laboratory is strongly discouraged for many reasons. If something serious happens, you may not be able to self-rescue. Your judgment may be impaired; for example, if you have a major spill or even a minor emergency, fatigue may impede or adversely affect your response.
2.6 High School Students
Proper supervision is the responsibility of the host Principal Investigator.
High school students must be registered through the NU Human Resources volunteer system and must complete the liability waiver required by Risk Management. For access to registry and safety training, unpaid high school students must be assigned a NetID and Person Outside the Institution (POI) number.
Assigned science projects should be commensurate with existing knowledge, skills and abilities. High school students cannot work with potential pathogens, radioactive materials or particularly toxic or reactive chemicals.
High school students must be properly supervised at all times and not allowed unaccompanied access or work in the laboratory.
Appropriate training, dress, lab coat and use of personal protective equipment is expected.
2.7 Research Safety
Research Safety helps faculty, staff, students, and visitors to work safely, to create safe workplaces, and to achieve and maintain compliance related to health, safety, and protection of the environment.
In carrying out this mission, Research Safety performs a basic risk management function in facilitating protection of the University and individual interests against loss from injury, accident, civil or criminal penalties, and litigation.
Research Safety provides information, training, and technical resources to department heads, center directors, and principal investigators to assist them in implementing the chemical, radiation, laser and biological safety programs.
Research Safety’s responsibilities for laboratory safety include:
- Compiling chemical inventory information and submitting reports to federal, state, and local agencies
- Collecting and disposing of waste and surplus chemicals
- Surveying laboratory facilities and offering recommendations for improved practice
- Maintaining records of laboratory facilities in Lumen
- Coordinating the registration programs required by the Institutional Biosafety Committee and the Radiation Safety Committee
- Providing 24-hour emergency response to spills or other accidents and investigating incidents involving hazardous materials
- Advising University personnel in safe work practices, personal protective clothing and equipment, engineering controls, and regulatory requirements
- Conducting or arranging for environmental monitoring
- Inspecting chemical fume hoods and other engineering controls
- Recommending policies and procedures for the safe conduct of work with chemicals
Research Safety representatives are authorized to enter University facilities within their jurisdiction at any time to observe working conditions, monitor equipment, and sample for contaminants. The director and the chemical hygiene officer are authorized to close a facility or stop a process or procedure that poses an imminent danger to life or property.
2.8 Committees
2.8.1 Laboratory and Chemical Safety Committee
A Laboratory and Chemical Safety Committee may convene ad hoc for cause. Members are drawn from each campus and a variety of disciplines, including chemistry, engineering, and biomedical sciences respectively. They are appointed by the vice president for research.
The committee’s responsibilities do not include research involving ionizing and non-ionizing radiation, recombinant DNA molecules, human blood, or pathogenic microorganisms. Such activities are under the jurisdictions of the Radiation Safety Committee, the Laser Safety Committee and the Institutional Biosafety Committee. Research involving animals is under the jurisdiction of the Institutional Animal Care and Use Committee.
The general purposes of the committee are to:
- Formulate and recommend to the vice president for research policies governing the use of chemical carcinogens and other chemicals in the laboratory
- Monitor the compliance of the University with respect to federal, state, and local regulations pertaining to hazardous materials in the laboratory
The Committee has a number of specific responsibilities, including:
- Recommending policies and procedures for a chemical safety program, including, but not limited to, educational programs, laboratory inspections, containment requirements, waste disposal programs, and medical surveillance
- Reviewing incident reports
2.8.2 Institutional Biosafety Committee (IBC)
All regulatory and safety issues related to the use of recombinant DNA, human blood, select agents, pathogenic microorganisms and biosafety level 3 laboratories are governed by the Institutional Biosafety Committee.
2.8.3 Radiation Safety Committee
All regulatory and safety issues related to the use of radioactive materials and X-ray are governed by the Radiation Safety Committee.
2.8.4 Laser Safety Committee
All regulatory and safety issues related to the use of Class 3b and 4 lasers are governed by the Laser Safety Committee. For licensing requirements, policies, and procedures see the Laser Safety Handbook.
3.0 General University Emergency Information
In University buildings, always call 911 if there is an explosion, fire, injury, or spill-related evacuation. If calling from a cell phone, report the incident’s building address, which is posted in each laboratory.
If applicable, know the appropriate emergency procedures for non-University locations. Call for assistance when needed. If there is a chemical, radioactive or biological material spill beyond the laboratory member’s ability to safely contain or clean up, call University Police at 456 at any time, and that office will contact Research Safety. During business hours, you may call Research Safety directly at 3-8300 (Chicago) or 1-5581 (Evanston).
3.1 University Emergency Response Plan
The University Police maintains the University’s Emergency Response Framework for emergencies. The Emergency Response Framework formalizes responses to all classes of emergencies, from small events to catastrophes. In emergency situations, the role of UP is to investigate the situation, provide site security, implement the emergency plan, and establish communications. Research Safety will advise and assist with hazardous-material spill control and cleanup. When the ability to respond adequately to an emergency is beyond the capability of the University personnel, UP will call the local fire department or local hazardous materials response team. Research Safety may direct UP to make this call.
3.2 Building Emergency and Evacuation Plans
The University Emergency Response Framework requires that department heads cooperate to establish building safety committees and appoint building safety managers and alternates. The building safety committees develop evacuation plans for each building. The plans include a telephone tree for notifying key persons in case of emergency. All building occupants receive training in their respective evacuation plan. Safety wardens are appointed for each building.
In the event of a fire, hazardous material release, or other hazardous situation requiring emergency response in a safety warden’s zone, the warden will:
- Activate the fire alarm, if needed
- Call University Police and report the incident
- Notify occupants to evacuate the zone
- Assist emergency personnel by providing information regarding location of the incident, origin, and persons involved
The senior officials of Research Safety, University Police, Risk Management, and Facilities are authorized to initiate evacuation of buildings.
3.3 Loss of Power4
Most laboratory buildings experience occasional brief periods of power loss. Such instances may be minor disturbances or could damage equipment or ruin experimentation. Longer term power outages may cause significant disruption and loss. It is prudent to consider the effects of long-term and short term power loss and implement plans to minimize negative outcomes.
3.3.1 Short-Term Power Loss
Consider what can happen in the event of short-term power loss. If the outcome is more than just an inconvenience, implement steps to reduce the impact. For example, if temperature is regulated by a heating mantle and loss of heat for even a few minutes could create an unacceptable variation, the result may be loss of that particular experimental run.
When developing a plan for handling a short-term power loss, consideration should be given as to what state a piece of equipment goes to during a loss of power or a resumption of power. Equipment should enter a fail-safe state and it should be tested for this state by purposely shutting off power to it and then reenergizing the circuit. Any interlocks (e.g., against high temperatures on heating mantles) should be rechecked after a loss of power. Some equipment must be restarted manually after a shutdown, resulting in longer term power loss even when power is restored. Uninterruptable power supplies and automatic generators should be considered for freezers and refrigerators that are used to store unstable compounds.
Laboratory Procedures
If laboratory personnel are present when power is lost, and power is not restored immediately, consider the following actions:
- Turn off equipment, particularly if leaving before power is restored. Some equipment can be damaged if turned on abruptly once power comes back online. If no one is in the laboratory when the power is restored, equipment that does turn on will be running unattended.
- Discontinue operations requiring local ventilation, such as laboratory chemical hoods. The building ventilation system may not be on emergency power.
- Close laboratory chemical hood sashes.
3.3.2 Long-Term Power Loss
Damaged power distribution systems and other conditions may result in power loss that lasts hours or days. This has implications for security, safety, and experimental work that go well beyond those for a short-term power loss.
Security Issues
For laboratory spaces with specialized security systems, such as card readers or electronic locks, know if the locks are locked or unlocked in the event of power failure. Develop a backup plan for laboratory security in the absence of such systems.
Environmental and Storage Conditions
The most common problem during a power outage is storage of materials that require specialized environmental conditions, such as refrigeration and humidity controls. For example, sub-80 C freezers, may hold their temperature for a few hours after a power loss but will eventually warm. This warming may lead to loss of samples or, for materials that become unstable when warmed, to more hazardous conditions, including fire, over pressurization, or release.
Discontinuation of Experiments
Experiments that rely on power may need to be discontinued and disassembled. Leaving the materials in place may not be prudent. Assign responsibility to identify problems and ensure that materials are safely stored.
Preplanning
There are many options for minimizing the effects of a power loss, including alternative energy sources and, when that is not practical, prioritizing experimental needs, consolidating, and using dry ice. Do not depend on safety showers or eyewashes. This safety equipment relies on a booster pump that may not be operational. Emergency telephones and manual pullbox stations should continue to operate properly. Be prepared by keeping flashlights in the work area.
Generator Power
A laboratory building may be connected to an emergency generator. If so, know what circuits or outlets are supported by emergency power. In some buildings, for example, the generator may only run emergency lighting and security systems. In others, power for the ventilation system, all or in part, may be backed up by the generator. Some buildings may have specially marked outlets that are connected to the generator. One potentially negative aspect of a generator is that there is usually a slight delay, up to several seconds, from the time the power is lost to the time that the power load is taken up by the generator. Equipment that is sensitive to a minor power disruption may be affected and a generator may not provide power without an interruption.
Know what will continue to operate during a power loss. Determine how long the laboratory operation can rely on the generator. If there is equipment that would benefit from generator backup, inquire about the making of such a connection.
Uninterruptible Power Supply (UPS)
When generator power is not available or if equipment is sensitive to the slight power delay, UPS systems may be the right choice for continued power. UPS systems are composed of large rechargeable batteries that immediately provide emergency power when the main supply is interrupted. UPS systems come in a variety of types and sizes. The three basic types are offline, line interactive, and online. The differences among the three are related to the level and type of surge protection, with the offline providing the least amount of surge protection and the online providing the most sophisticated protection. Size varies based on power needs. When purchasing an UPS for equipment other than a computer, consult with the equipment manufacturer to help choose the right solution. All UPS systems require some degree of maintenance. The battery needs to be replaced at an interval specified by the manufacturer. Batteries may be expensive and should be figured into the cost of the system.
3.4 Floods5
Floods could be due to rain, water pipe breaks, or accidental or deliberate acts. Some areas are more prone to flooding than others. Laboratory spaces on the basement or ground level are more likely to be flooded in a storm than those on higher floors. Safety showers and eyewash stations that are not properly plumbed or do not have floor drains nearby may also be a source of flooding. Consider the likelihood of flooding and its impact. Also consider whether the laboratory space contains equipment that is very sensitive to water damage. If flooding occurs, could it affect the space below the flood? If so, is the floor sealed appropriately? Are there overhead pipes?
To avoid flooding, do not block the sink drains. Place rubber matting in the bottom of the sinks to prevent breakage of glassware and to avoid injuries. While the use of water as a coolant in laboratory condensers and other equipment remains common practice, there are alternative means for example a FindenserTM. Many flooding incidents occur when the tubing supplying the water to the condenser disconnects. Hoses can pop off when building water pressure fluctuates, causing irregular flows, or can break when the hose material has deteriorated from long-term or improper use. Floods also result when exit hose jump out of the sink from a strong flow pulse or sink drains are blocked by an accumulation of extraneous material. Proper use of hose clamps and maintenance of the entire cooling system or alternative use of a portable cooling bath with suction feed can resolve such problems.
3.5 Incident (Accident) Reporting
Laboratory incidents shall be investigated. The PI shall submit an Incident Report in case of injury, minor spills, fires, hazardous material releases, or near misses. In the event of a work related injury the PI follows the Occupational Health protocol for the respective campus: Lab Injury Protocol – Evanston or Lab Injury Protocol – Chicago and fill out a Supervisor’s Injury or Illness Investigation Report .
Research Safety may ask for safety committee assistance to investigate and prepare an investigation report. Investigations are made and reports written not only to satisfy certain laws but more importantly to learn the cause of the problem and what changes in procedures, equipment, or training should be made to avoid other accidents.
All lost time claims shall be reported to Occupational Health. Work related injuries are entered in the OSHA Injury Log. In case of a fire, injury, or other accident requiring outside assistance, the Safety and Loss Prevention Division may write an investigation report.
3.5.1 Research Safety Assistance
Research Safety will respond to chemical, radioactive and biological materials spills. However, if the spilled material is not volatile and there is no immediate fire or toxic hazard, cleanup may be done by laboratory members (under direction of the PI or Research Safety). Research Safety will provide cleanup supplies and equipment, and cleanup instructions. In situations involving a fire of research chemicals or toxic hazards, Research Safety will advise on evacuation or other precautions to protect persons or property in the immediate area.
3.6 Personal Injury
At least two members of each lab group should receive first aid and CPR training. PIs/supervisors must determine whether to arrange for and/or sponsor first aid and CPR training for their staffs. In the event of a work related injury follow the Occupational Health protocol for the respective campus: Lab Injury Protocol – Evanston or Lab Injury Protocol – Chicago.
3.6.1 Burn from Fire
- If your clothing catches fire, immediately get under a safety shower or other water source.
- Assess the condition of the skin’s burn area. If skin is not broken, run water over the burn area to remove heat. Don’t put ice on the burn. If skin is broken, apply a dry, sterile dressing over the wound.
- Seek medical attention as soon as possible.
3.6.2 Inhalation
A person exposed to smoke or fumes shall be removed to fresh air. Any victim overcome by smoke or fumes shall be treated for shock. Call 911. Give cardiopulmonary resuscitation (CPR) if necessary and if trained personnel are available. If a person needs to be rescued from a contaminated area, evaluate the possibility of harm to the rescuer before anyone enters or remains in the contaminated area without proper protective equipment. If a printed SDS is available for the material inhaled, it should accompany the victim to the medical treatment facility.
3.6.3 Shock
Shock is likely to develop in any serious illness or injury. Shock is a condition in which the circulatory system fails to deliver blood to all parts of the body. When the body’s organs do not receive adequate blood supply, they fail to function properly. The following signals are indicators that the victim is suffering from shock:
- Restlessness or irritability (often the first sign that the body is experiencing a significant problem)
- Altered consciousness
- Pale, cool, moist skin
- Rapid breathing
- Rapid pulse
In caring for shock, have the victim lie down. Help the victim rest as comfortably as possible to minimize pain and thereby slow the progression of shock. Control any external bleeding. Help the victim maintain a normal body temperature and avoid chilling. Elevate the victim’s legs about 12 inches unless you suspect broken bones or possible head, neck, or back injuries. If in doubt, leave the patient lying flat.
Do NOT give the victim anything to eat or drink although they may complain of thirst. Obtain medical assistance promptly since shock cannot be managed by first aid alone.
3.6.4 Ingestion
If a person ingests a toxic chemical, determine, if possible, what was ingested and notify the emergency medical personnel. Contact the Poison Control Hotline at (800) 222-1222 for emergency response information for the specific compound.
Inform the hotline personnel of the first aid treatment shown on the container label or the SDS. The printed SDS should accompany the victim to the medical treatment facility.
3.6.5 Puncture or Cut
When treating a victim with a puncture wound or cut, wear personal protective equipment (e.g., gloves) to minimize exposure to human blood, body fluids, or other chemical or biological contamination. Apply a pressure pad or clean cloth firmly to the wound. Raise the wounded area above the level of the heart to slow the bleeding. For severe bleeding or spurting, very firmly press a pressure pad directly on the wound and apply pressure at the applicable body pressure point above the wound to stop the flow of blood. In a severe injury, keep the victim warm, calm, and oriented to prevent shock.
3.6.6 Needlestick
Immediately wash needlesticks or other accidents involving skin punctures by a chemical or biological agent. Appropriate medical testing, treatment, and follow-up may be indicated and shall be provided as appropriate. See the Exposure Control Plan for more information on needlestick exposures to human blood and other potentially infectious human materials.
3.6.7 Dermal Contact
If a chemical spills on a person, the first goal is to remove the chemical from the person’s skin as soon as possible, without spreading it onto yourself. For chemicals that can cause burns, the stronger the chemical and the longer the contact, the worse the burn. The chemical continues to burn as long as it remains on the skin. For all chemicals except hydrofluoric (HF) acid (see section 5.1.1 First Aid Procedure for Responding to Hydrofluoric Acid Burns), flush the skin under running water or a safety shower for at least 15 minutes. For limited skin exposure on a small area, a drench hose may be adequate for flushing.
Remove contaminated clothing while the person is under the shower stream, taking care not to spread contamination from the clothing onto more of the person’s skin. If the clothing must be pulled over the head or down along the legs to be removed, cut it away with first aid kit scissors instead. Many safety showers are equipped with curtains to give privacy to the victim. Don’t let modesty keep you from removing contaminated clothing that remains against skin.
Do not treat the burn. Do not puncture any blisters that may develop. Allow trained medical personnel to administer treatment after flushing is complete. Your first aid kit will probably contain antibiotic ointment and sterile gauze for burns. These are intended only for minor burns such as those you might encounter in your household, e.g., small burns from cooking at a stove and sunburns.
It is advisable for pregnant women to avoid touching anything in a laboratory space bare-handed. Disposable gloves provide a barrier from low level contamination of common surfaces.
3.6.8 Eye Contact
Should a chemical enter a person’s eye(s), wash the eye(s) with running water for at least 15 minutes, while waiting for medical help to arrive. Keep the affected eye (if only one has been contaminated) lower than the unaffected eye to prevent the spread of contamination.
Be aware that particulates and liquids can become trapped in the conjunctiva where they may continue to cause damage. The entire interior of the eye socket must be flushed as well as the exposed cornea.
A “buddy” in the laboratory space is vital to the injured person to help find the eyewash, call for help, keep the eyes open under the water stream, and prevent the person from rubbing the eye(s) and aggravating the damage.
3.7 Critical Failures
Critical failures in research processes or research equipment require immediate action towards cessation. Examples include:
- Unsafe processes that led to work-acquired injury–permanent eye damage or hospitalization
- Apparently work-acquired infection or possibly work-acquired high contaminant levels found in body fluids, hair, etc.
- Any containers for flammables, reactives, toxics that show evidence of rupture in storage (broken cap, vessel rupture)
- Toxic or highly toxic gas cylinders mounted in use outside of a vented enclosure. In-use cylinders of this content type should be mounted for use in a vented gas cabinet or fume hood.
- For high-pressure flammable gases: no flash arrestor downstream of gas cylinder to usage device
- In-use flammable or toxic gas cylinders with regulators where pressure is greater than the ASME B40.100 (accuracy classes from 0.1 to 5% range) out of spec.
- In-use flammable/toxic gas leakage alarm systems or waste abatement systems without PM/calibration records for last 12 months
- In-use flammable and toxic gas manifolds without an updated process flow diagram
- In-use flammable and toxic gas manifolds missing a check valve preventing accidental back flow conditions. Especially critical for oxy/fuel systems
- In liquid cryogenic flow systems a pipe/tube/vessel area/section that can be valved off without a safety relief valve in place
- Heating devices for flammable or toxic chemistry where the temperature gauge is >5% out of spec
- Inappropriate use of toxic and flammable gas fitting adapters (i.e., wrong CGA type)
- Use of cheater plugs or cheater adaptors to connect devices to a >=110V electrical power source
4.0 Laboratory Safety and Chemical Hygiene Framework
Laboratory safety and chemical hygiene programs consolidate the compliance programs for the OSHA Hazard Communication Standard, the OSHA Occupational Exposure to Hazardous Chemicals in Laboratories Standard (the “Laboratory Standard”), and other general laboratory safety programs.
4.1 Lumen and the Research Safety Website
PIs use the Research Management Platform Lumen to submit applications and registrations for review. Lumen helps PIs build a lab-specific laboratory-spcific profile and serves as an educational resource for PIs and laboratory members.
The information in Lumen and the Research Safety website is intended to be a central safety resource for the laboratory, shop, and department.
A PI’s Lumen Profile is the laboratory-specific part of the chemical hygiene plan required by the OSHA Laboratory Standard for research labs, teaching labs, and common facilities (those shared by more than one researcher). In the case of shared facilities, the director, coordinator, or designated facility supervisor is responsible for their Lumen Profile.
If chemical, radioactive or biological agents or processes with lasers, physical or health hazards are used, the PI shall request a Lumen Profile.
PIs are responsible for keeping their Lumen Profile current. At a minimum, a review and update should be conducted annually and when there are changes to personnel, space or required registrations. The Laboratory Safety Profile shall reflect current job activities which affect occupational exposure.
If a PI opens a new laboratory space or moves to an alternate location, advise Research Safety.
4.2 Laboratory Inspections
The University provides an inspection program for all laboratories. Laboratory inspections are conducted by Research Safety staff.
The inspection consists of an interview with the laboratory representative followed by a visit to the laboratory. Investigators may be asked to update their Lumen Profile and other information. The Research Safety representative may examine general laboratory conditions, engineering controls, work practices, chemical storage, use of personal protective clothing and equipment, signs and postings, and availability of documents such as SDSs. Laboratory members may be interviewed. Inspection findings are detailed in the Lumen Profile. If corrections are needed, PIs are required to respond within two weeks.
5.0 General Laboratory Safety
Working safely in a laboratory requires having the proper containment equipment and engineering controls, wearing appropriate personal protective equipment, using proper work practices, knowing safety information for the materials and equipment used, and following safety instructions and laboratory protocols.
Because each laboratory situation is different, judgment is required in interpreting general concepts for individual settings. The Lumen Profile provides specific information for individual laboratories.
5.1 First Aid Kits
Medical care at the University is available through the University Health Service, the occupational medicine providers for each respective campus, and local hospitals. On the campuses, a 911 call summons paramedics within minutes.
PIs are responsible for supplying at least one first aid kit for their lab groups. If the same group occupies spaces that are not in immediate proximity (i.e., labs in different buildings or on different floors), a first aid kit shall be available for each set of labs.
First aid kits and first aid training are also required for research activities away from the campus especially in remote areas where medical care is not readily available.
Factors to consider in selecting a kit include the following:
- The supplies should be consistent with the types of injuries anticipated in this research space (e.g., will there be burns, cuts, fractures, contusions, or allergic reactions).
- Supplies should be provided in single-use or -dose unit-type packs with suitable wrapping to ensure sterility and hygiene.
As a practical model, the American National Standards Institute’s Minimum Requirements for Workplace First Aid Kits (ANSI Z308.1-1998) recommends that basic units should contain:
- 1 absorbent compress (32 sq. in. with no side smaller than 4 in.)
- 16 adhesive bandages (1 x 3 in.)
- Adhesive tape (total of 5 yd.)
- 10 individual-use antiseptic applications (0.5 g each)
- 6 individual-use burn treatment applications (0.5 g each)
- 2 pairs of medical exam gloves
- 4 sterile pads (3 x 3 in.)
- 1 triangular bandage (40 x 40 x 56 in.) and
- 1 scissors
It shall be inspected regularly to ensure that no items are missing and that none of the remedies (e.g., saline solution, ointment) in the kit have expired.
The LCSC and Research Safety encourage CPR and first aid training for at least 2 lab members in each lab group. Such training can be arranged through Research Safety.
If there are lab members who have particular sensitivities or medical problems that could interfere with first aid procedures, consider discussing this issue with the entire staff. Barring any confidentiality concerns, it is wise to prepare colleagues for possible reactions or symptoms should an employee suffer from an illness that demands special care. An employee with a given medical condition (e.g., severe allergies, asthma, heart disease) may require prescription drugs during a respiratory attack or illness episode.
5.1.1 First Aid Procedure for Responding to Hydrofluoric Acid Burns
Hydrofluoric acid (HF) is an extremely hazardous liquid. It can cause severe skin and eye irritation or deep-seated, slow-to-heal burns. In certain cases, exposure can prove fatal. For any major exposure to HF, immediate paramedic assistance is necessary.
HF’s mode of action is to bind calcium whenever contact occurs with skin or other body tissues. Unlike the action of other acids, which are rapidly neutralized, tissue destruction and action of HF may proceed for days. Because calcium is necessary for cell life, its binding can bring about rapid cell death. If the HF exposure is extensive, excessive amounts of calcium may be inactivated and inadequate supplies of calcium may be available for vital bodily functions.
Some fluorinated compounds may generate HF either as a reaction side-product or as a result of decomposition. Labile fluorine in fluorinated organic or inorganic materials may produce HF, particularly through contact with water. Fluoride ions also form HF when in equilibrium in aqueous solution. Researchers should consult the SDS of the compound with which they are working and the scientific literature during their preliminary risk assessment to identify whether HF formation is a possible hazard.
Inform the physician treating the HF injury to the nature of the chemical involved in the exposure and deliver a SDS. Some medical providers may not commonly encounter HF. Offer as much information as possible regarding the chemical and its effects. Encourage the physician to consult an occupational specialist for further information, if needed. See also the CDC’s HF page.
For skin exposure 6:
- Immediately start rinsing under safety shower or other water source and flush affected area thoroughly with large amounts of water, removing contaminated clothing while rinsing. Speed and thoroughness in washing off the acid is of primary importance.
- Call for emergency response.
- While wearing neoprene or butyl rubber gloves to avoid a secondary aqueous HF burn, massage 2.5% (w/w) calcium gluconate gel onto the affected area after 5 minutes of flushing with water. If calcium gluconate gel is unavailable, continue flushing the exposed areas with water until medical assistance arrives.
- Send a copy of the SDS with the victim.
For eye exposure:
- Immediately flush the eyes, holding eyelids open, for at least 15 minutes with large amounts of gently flowing water, preferably using an eyewash station.
- Do not apply calcium gluconate gel directly onto the eye.
- Seek medical attention.
- Send a copy of the SDS with the victim.
For inhalation:
- Immediately move to fresh air.
- Call 911.
- Send a copy of the SDS with the victim.
For ingestion:
- Seek immediate medical attention.
- Drink large amounts of water or milk as quickly as possible to dilute the acid.
- Do not induce vomiting. Do not ingest emetics or baking soda. Never give anything by mouth to an unconscious person.
- If medical attention must be delayed and the materials are available, drink several ounces of milk of magnesia or other antacids.
- Send a copy of the SDS with the victim.
PIs should assign and require completion of Research Safety developed training content. Calcium gluconate gel (2.5% w/w) must be readily accessible in work areas where any potential HF exposure exists. Check the expiration date of your supply of commercially obtained calcium gluconate gel and replace as needed to ensure a supply of fresh stock. Research Safety provides calcium gluconate gel for labs working with HF and HF-generating materials.
5.2 Personal Hygiene
Personal hygiene is extremely important in a laboratory. Contamination of food, beverages, or smoking materials is a potential route of exposure to toxic chemicals, radioactive materials, or biological agents through ingestion. Thus, laboratory personnel shall not prepare, store, or consume food or beverages, pipette by mouth, smoke, apply lip balm or cosmetics, or handle contact lenses in the work area. This elementary safety rule shall be followed by everyone working in or visiting a laboratory space.
Hand washing is a primary safeguard against inadvertent exposure to toxic chemicals, radioactive materials or biological agents. Always wash your hands before leaving the laboratory space, even though you use gloves. Wash your hands after removing soiled protective clothing, before leaving the laboratory space, and before eating, drinking, smoking, or using a rest room.
Wash your hands periodically during the day at intervals dictated by the nature of your work. Wash with soap and running water, with hands held downward to flush the contamination off the hands. Turn the tap off with a clean paper towel to prevent recontamination and dry your hands with clean towels.
Confine long hair and loose clothing when in the laboratory or in a shop area to keep them from catching fire, dipping into chemicals, or becoming entangled in moving machinery. Avoid wearing finger rings, fingernail extensions and wrist watches which may become contaminated, react with chemicals, or be caught in the moving parts of equipment.
Remove contaminated laboratory coats and gloves before you leave the laboratory. Keep a clean spare coat to wear outside the laboratory. Do not wear gloves outside the laboratory.
5.3 Personal Protective Clothing and Equipment
You have a responsibility to dress sensibly for laboratory work. Some protection is afforded by ordinary clothing and eyeglasses.
Personal protective clothing and equipment protects you from injury due to absorbing, inhaling, or coming into physical contact with hazardous materials. You are responsible for using special protective clothing and equipment when they are required for safety. Protective wear may include laboratory coats, wraparound gowns, cloth masks, coveralls, aprons, gloves, shoe covers, and respirators. Select garments and fabric based on the nature of the hazardous agent. Laboratory workers must check their PPE for signs of wear or damage and replace the affected equipment immediately.
Research Safety provides a basic set of PPE – lab coat, gloves and eye protection – to all registered laboratory workers. The standard lab coat fabric is 100% cotton. Flame-resistant (FR) lab coats provide additional protection when working with flammable chemicals or processes. FR-rated clothing has a distinctive label affixed to it.
Research Safety facilitates lab coat laundry service. Simply bring a dirty lab coat to the Evanston Lab Coat Drop-off (near the Fisher Stockroom) or the Chicago Fisher Stockroom. You will receive a clean one of the same size. For those who wish to have their own personal lab coat returned to them, you will need to have it cleaned through Procurement and Payment Services and your department assumes the cost.
Do not wash lab coats or contaminated clothing with other personal laundry.
5.3.1 Clothing
Cover unprotected skin whenever possible. Suitable clothing shall be worn in the laboratory space; shorts are not appropriate. Clothing may absorb liquid spills that would otherwise come in contact with your skin. Long sleeves protect arms and shall fit snugly, especially when you are working around machinery. Nomex and wool affords more protection from flash burns or corrosive chemicals than cotton or synthetic fabrics. Some synthetic fabrics may increase the severity of injury in case of fire. Cotton is less prone to static electricity buildup than nylon or other synthetics.
Wear substantial closed-toed shoes in the laboratory space to protect against chemical splashes or broken glass. Do not wear sandals, cloth sport shoes, perforated shoes, or open-toed shoes. If you clean up a spill from the floor, you may need the added protection of rubber boots or plastic shoe covers. Steel-toed shoes may be required for handling heavy items, such as gas cylinders or heavy equipment components.
Aprons, laboratory coats, gloves, and other protective clothing, preferably made of chemically inert material, shall be readily available and used. Laboratory coats are essential to protect street clothing from biological agent aerosols or chemical and radioactive material splashes and spills, vapors, or dusts. For work involving carcinogens, disposable coats may be preferred. For work with mineral acids, acid-resistant protective wear is desirable.
When the potential for fire exists, consider wearing a laboratory coat specifically designed to be flame retardant. Several types of flame-resistant clothes are available from safety suppliers. A low-cost option is a disposable cotton coat that has been treated with a flame-resistant material. The treatment slows combustion and provides an additional level of protection from fire and heat. However, repeated washing degrades the chemical treatment and compromises fire protection.
More durable flame-resistant cotton laboratory coats are also available. A fabric known as Nomex provides the best protection against flame hazards. This material has a structure that thickens and carbonizes when exposed to heat. This unique characteristic gives Nomex lab coats excellent thermal protection. Because the characteristics of the material are inherent to the fiber, repeated laundering does not change the thermal protection capabilities.
5.3.2 Eye Protection
Eye protection is mandatory in laboratory spaces because of the obvious hazards of flying objects, splashing chemicals, and corrosive vapors. Eyes are very vascular and can quickly absorb many chemicals. Regulations require protective eye and face equipment where there is a reasonable probability that using them can prevent injury. Eye protection shall be required in all laboratories where chemicals are used or stored. Eye protection is not interchangeable among employees and shall be provided for each individual unless disinfected after use.
Safety glasses with clear side shields are adequate protection for general laboratory use. Goggles shall be worn when there is danger of splashing chemicals or flying particles, such as when chemicals are poured or glassware is used under elevated or reduced pressure. A face shield with goggles offers maximum protection (for example, with vacuum systems that may implode).
Corrective lenses in spectacles do not in themselves provide sufficient protection. Wear goggles over your eyeglasses, or order prescription safety glasses through Eyelation. Online Eyelation access for Northwestern University faculty, staff and students is available through the Research Safety offices.
TABLE 5.3.1 PROPERTIES OF PROTECTIVE CLOTHING MATERIALS*
| MATERIALS | STRENGTH | CHEMICAL RESISTANCE | FLAMMABILITY | STATIC PROPERTIES | COMFORT | USES |
|---|---|---|---|---|---|---|
| COTTON | Fair durability | Degraded by acids; binds | Special treatment for flame | No static problems | Comfortable, lightweight | Lab coats |
| MODACRYLIC | Resistant to rips and tears but less so than polyamide fibers; abrasion-resistant but less so than nylon or polyester | Resistant to most chemicals | In direct flame, fabric shrinks to resist flame penetration; will not melt or drip; self-extinguishing; rapidly dissipates when source of ignition is removed | Has antistatic properties | Comfortable, soft, and resilient; easy to clean; has soil release properties | Lab coats |
| NYLON | Exceptionally strong and abrasion resistant | Not water absorbent | Melts when heated; requires flame retardant | Static buildup possible; requires antistatic agent | Lightweight | Lab coats |
| PLASTIC | Usually reinforced at points of strain; will not stick together, peel, crack, or stiffen | Resistant to corrosive chemicals | Can be ignited by flammable solvents and others in event of static discharge | Accumulates considerable charge of static electricity | Lightweight | Aprons, sleeve protectors, boots |
| POLYOLEFIN | Resistant to rips and tears | Excellent chemical resistance; low binding for chemicals | High melting point; flame-resistant | Good static dissociation | Lightweight; good permeability; limited moisture absorbency; wearer perspiration may cause discomfort | Bouffant caps |
| POLYPROPYLENE | Strong | Resistant to most chemicals; oxygen and light-sensitive | Low melting point; requires flame retardant | Static buildup; requires antistatic agent | Lightweight | Aprons |
| RAYON | Fairly durable | Degraded by acids; binds some chemicals | Lab coats |
* Based on manufacturer’s claims. From Chemical Safety Manual for Small Businesses, American Chemical Society, third edition, 2007.
5.3.3 Gloves
Gloves are worn to prevent skin contact with toxic, radioactive or biological agents, burns from hot or extremely cold surfaces or corrosives, or cuts from sharp objects. Glove usage and artificial fingernail extensions are not compatible. Many gloves are made for specific uses. For adequate protection, select the correct glove for the hazard in question.
Leather and Kevlar gloves provide good protection for picking up broken glass, handling objects with sharp edges, and inserting glass tubing into stoppers. Cuts from forcing glass tubing into stoppers or plastic tubing are a common laboratory accident and are often serious. However, because they absorb liquid, these gloves do not provide protection from chemicals, nor are they adequate for handling extremely hot or cold surfaces. Gloves designed to insulate against hot surfaces and dry ice are not suitable for handling other chemicals.
Sometimes the ideal glove is actually two gloves worn together. Wearing one pair of gloves (such as reusable nitrile, neoprene, butyl, or Viton) over a flexible laminate combines the advantages of both.
7When choosing an appropriate glove, consider the required thickness and length of the gloves as well as the material. Consult the glove manufacturer for chemical-specific glove recommendations and information about degradation and permeation times. Certain disposable gloves should not be reused.
- Butyl is a synthetic rubber with good resistance to weathering and a wide variety of chemicals.
- Natural rubber latex is a highly flexible and conforming material made from a liquid tapped from rubber plants. Use of latex is not recommended as it can cause allergic reactions.
- Neoprene is a synthetic rubber having chemical and wear-resistance properties superior to those of natural rubber.
- Nitrile is a copolymer available in a wide range of acrylonitrile content; chemical resistance and stiffness increase with higher acrylonitrile content.
- Polyethylene is a fairly chemical-resistant material used as a freestanding film or a fabric coating.
- Poly (vinyl alcohol) is a water-soluble polymer that exhibits exceptional resistance to many organic solvents that rapidly permeate most rubbers.
- Poly (vinyl chloride) is a stiff polymer that is made softer and more suitable for protective clothing applications by the addition of plasticizers.
- Polyurethane is an abrasion-resistant rubber that is either coated into fabrics or formed into gloves or boots.
- 4H® or Silvershield® is a registered trademark of North Hand Protection; it is highly chemical-resistant to many different classes of chemicals.
- Viton®, a registered trademark of DuPont, is a highly chemical-resistant but expensive synthetic elastomer.
Chemicals can eventually permeate all glove materials. Select glove materials resistant to the chemical being used, and change gloves periodically to minimize penetration. The chemical resistance of common glove materials varies according to the glove manufacturer, as manufacturers may vary the thicknesses and formulations of materials. Consult Research Safety for concerns regarding glove selection.
General guidelines to the selection and use of protective gloves:
- Do not use a glove beyond its expiration date. Gloves degrade over time, even in an unopened box.
- When not in use, store gloves in the laboratory space but not close to volatile materials. Do not don or doff gloves in offices, in break rooms, lunchrooms or common hallways.
- Inspect gloves for small holes, tears, and signs of degradation before use.
- Replace gloves periodically because they degrade with use, depending on the frequency of use and their permeation and degradation characteristics relative to the substances handled.
- Replace gloves immediately if they become contaminated or torn.
- Decontaminate or wash gloves appropriately before removing them. [Note: Some gloves, e.g., leather and poly (vinyl alcohol), are water permeable. Unless coated with a protective layer, poly (vinyl alcohol) gloves will degrade in the presence of water.]
- Gloves on a glovebox should be inspected with the same care as any other gloves. Disposable gloves appropriate for the materials being handled within the glovebox should be used in addition to the gloves attached to the box. Protect glovebox gloves by removing all jewelry prior to use.
5.3.4 Respirators
When feasible, engineering controls shall be provided to minimize exposure to airborne hazards. If accepted engineering control measures are not available to prevent or protect against harmful levels of airborne contaminants, employers are required to provide respirators at no cost to employees and employees are required to wear them. Respirators are considered a last resort of protection against exposure to inhalation hazards after all practicable engineering options have been exhausted.
Persons desiring to use a respirator shall inform Research Safety and obtain information on the requirements. These requirements are mandated by the OSHA Respiratory Protection Standard and are described in the University’s Respiratory Protection Program.
A hazard evaluation shall be conducted to determine whether the employee or student is required to wear a respirator or whether engineering controls can eliminate the hazard. If the need for a respirator is established, or if a researcher wishes to wear a respirator on a voluntary basis, the wearer must register with Research Safety.
5.4 General Laboratory Protocol
All laboratory protocols shall include basic safety precautions. These include personal hygiene, work practices, and the appropriate personal protective clothing and equipment needed to protect from exposure to chemicals, radioactive materials or biological agents.
5.4.1 Housekeeping
Wise up, suit up, clean up!
Keeping things clean and organized helps provide a safer laboratory space. Laboratory surface cleanliness is especially important for laboratory workers of reproductive age and pregnant women. Keep drawers and cabinet doors closed and electrical cords off the floor to avoid tripping hazards. Keep aisles clear of obstacles such as boxes, chemical containers, and other storage items that might be put there even temporarily. Avoid slipping hazards by cleaning up spilled liquids promptly and keeping the floor free of stirring rods, glass beads, stoppers, and other such items. Never block or even partially block the path to an exit or to safety equipment, such as a fire extinguisher or safety shower.
Make sure that supplies and equipment on shelves provide sufficient clearance so that fire sprinkler heads operate correctly. There shall not be any storage within 18 inches of a sprinkler head.
Put ordinary wastepaper and wrappings in a labeled trash can. When discarding empty boxes or other containers bearing hazardous materials labels, the labels shall be defaced or removed before disposal. Dispose of broken glass and other sharp items in a rigid, puncture-resistant container to protect persons collecting the waste materials. Needles and syringes must be disposed of in a rigid, puncture-resistant sharps container.
Chemical wastes and unwanted chemicals shall be disposed of promptly and not left to clutter a laboratory space. Follow all procedures of the Hazardous Waste Disposal Guide (Purple Guide). Additional information is available on disposal of human body fluids or other potentially infectious materials.
5.4.2 Cleaning Glassware
When cleaning laboratory glassware, wear appropriate cut-resistant gloves. Avoid accumulating too many articles in the cleanup area around the sink; space is usually limited and piling up glassware leads to breakage.
Many fingers have been badly cut by broken glass from glassware that was intact when put into the sink water. Handle glassware carefully and watch out for broken glass at the bottom of the sink. A rubber or plastic mat in the sink will help minimize breakage.
If you must use hazardous substances such as solvents or acids for cleaning glassware, you must be thoroughly familiar with those materials’ hazards and use appropriate protective equipment.
Avoid using strong cleaning agents such as nitric acid, chromic acid, sulfuric acid, strong oxidizers, or any chemical with “per” in its name (perchloric acid, ammonium persulfate, etc.) unless no alternatives are available.
Flammable solvents such as acetone should be used in minimum quantities for cleaning and with appropriate precautions taken during their use.
Acids and solvents shall not be rinsed down the drain during cleaning but shall be collected for proper treatment and disposal.
5.4.3 Laboratory Animals
Federal regulations require that the Institutional Animal Care and Use Committee (IACUC) review and approve the use of animals in research. The Center for Comparative Medicine (CCM) administers all activities related to the care and use of animals and mandates compliance with standard operating procedures.
Laboratory animals may be potential sources of hazardous chemical exposure from metabolic products, wastes, cage litter, and contaminated cages. The preparation of food and water containing toxic substances under investigation shall be done with all precautions ordinarily taken to protect the health and safety of personnel. The OSHA Laboratory Standard guidelines for animal work with chemicals of high chronic toxicity shall be followed. The guidelines cover administration of the toxic substance, aerosol suppression, personal protection, and waste disposal.
Another possible concern in handling laboratory animals is the potential for exposure to inherent biological hazards. Aside from the biological agents to which the animals are deliberately exposed, lab animals may harbor indigenous pathogens that can be transmitted to humans. This is of particular concern with nonhuman primates. In the case of macaque monkeys, animal handlers may contract Cercopithecine herpesvirus ([CHV-1], commonly referred to as Herpesvirus simiae or “B-virus”) infection that can be deadly. The virus is primarily transmitted through bites, scratches, or other contamination of broken skin; however, a fatality due to a splash of a macaque’s body fluid in the eye has been reported. The high risk of infection places particular importance on the wearing of personal protective equipment to prevent exposure. Animal handlers working with macaques and other nonhuman primates shall follow the standard operating procedures from CCM and the Training and Occupational Health Program required by IACUC. Always don appropriate gloves, surgical masks, splash goggles, and lab coats or other suitable covering that leaves no exposed skin or mucous membranes.
Access to gaseous anesthetics and the operation of anesthesia machines shall be limited to authorized users. Authorized users, in this context, shall have completed safety awareness training.
5.4.4 Relocating or Closing a Laboratory Space
Disposition of all unwanted chemicals is the responsibility of the PI. All chemicals that will not be relocated shall be listed on the Hazardous Waste Pickup Request. The PI remains responsible until all hazardous materials are removed. The department of record is responsible for the safe and lawful cleanup and disposition of all chemical, biological and radioactive materials that are abandoned.
The PI ensures that surfaces and equipment potentially contaminated with hazardous chemicals, radioactive materials or biological agents are decontaminated before the laboratory space is vacated. Accessible surfaces (chemical fume hoods, sinks, bench tops) should be cleaned, when practical, by the PI and staff. If this is not possible, an outside contractor specializing in the testing and cleaning of contaminated laboratory equipment should be contacted. The PI shall provide the contractor with thorough and accurate information pertaining to the past uses of the equipment.
To confirm that a vacated laboratory space is properly emptied of hazardous materials, decontaminated, and ready for new occupants, the PI or laboratory supervisor shall prepare the Laboratory Closeout Checklist. Should the PI fail to complete the items required on the form, the department becomes financially and administratively responsible for the safe disposition of the hazardous materials and the decontamination of work surfaces.
Research Safety offers a laboratory survey to any PI vacating a laboratory space to assist in identifying the tasks that must be finished for clearance of the space.
5.4.5 Transportation and Shipping of Hazardous Materials
The U.S. Department of Transportation (DOT) requires that a licensed hazardous materials transporter be employed if hazardous materials are transported on a public highway, air, or water. DOT also requires that all individuals offering a hazardous material for transport receive appropriate training. Follow the Hazardous Materials Shipping Policy for Laboratories. If in doubt, consult with Research Safety before shipping any hazardous chemicals, radioactive materials, or biological materials. Export control restrictions may apply.
5.4.6 Laboratory Doors
Fire and life safety codes may require that corridor doors be fire rated and equipped with door closers. Doors with door closers are generally kept closed at all times, unless the door release is tied into the building’s fire alarm system. Keeping laboratory doors to corridors closed helps ensure that ventilation systems work properly and maintain contaminant-containing pressure differentials between labs and corridors. This is especially important in newer buildings with sensitive energy conservation systems. Doors in internal laboratory suites may have less stringent door closing requirements.
Viewing windows in corridor halls shall be kept unobstructed unless there is hazardous laser energy that requires curtains or blocking.
5.4.7 Visitors to Laboratory Spaces
Do not allow visitors, including children and pets, in laboratory spaces where hazardous substances are stored or are in use or hazardous activities are in progress. Students from primary and secondary schools occasionally may enter laboratories as part of educational programs under carefully controlled and supervised conditions. Colleagues, prospective students, and others may be invited into laboratory spaces for legitimate academic and research purposes. Each individual working in a laboratory should prudently evaluate the risks to visitors, especially to persons of increased risk such as children and immune-suppressed individuals. Human Resources requires registration of volunteers and interns. Occupational Health requires a signed Volunteers and Visitors Lab Use Agreement.
CCM standard operating procedures require a signed Visitor Hazard and Declaration of Compliance Form.
5.5 General Laboratory Techniques
5.5.1 Static Electricity
Static electricity may be generated whenever two surfaces are in contact with one another. Examples are processes such as evaporation, agitation, pumping, pouring of liquids, or grinding of solids or powders. Equipment used in these operations shall be bonded and grounded to prevent static charges from accumulating on the containers. Blanketing with inert gas may also prevent sparks in equipment where flammable vapors are present. The buildup of static charge increases at low absolute humidity, as is likely in cold weather. Some common potential sources of electrostatic discharges are ungrounded metal tanks and containers, metal-based clamps, nipples, or wire used with non-conducting hoses, high-pressure gas cylinders upon discharge, and clothing or containers made of plastic or synthetic materials.
5.5.2 Centrifuges
If a tabletop centrifuge is used, make certain that it is securely anchored in a location where its vibration will not cause bottles or equipment to fall. Ensure that the disconnect switch is working properly and shuts off the equipment when the top is opened. Centrifuge rotors shall be balanced each time they are used. Securely anchor and shield each unit against flying rotors. Regularly clean rotors and buckets with non-corrosive cleaning solutions. Always close the centrifuge lid during operation, and do not leave the centrifuge until full operating speed is attained and the machine appears to be running safely without vibration. Stop the centrifuge immediately and check the load balances if vibration occurs. Check swing-out buckets for clearance and support.
5.5.3 Vacuum Work and Apparatus 8
Vacuum work can result in an implosion and the possible hazards of flying glass, spattering chemicals, and fire. Set up and operate all vacuum operations with careful consideration of the potential risks. Although a vacuum distillation apparatus may appear to provide some of its own protection in the form of heating mantles and column insulation, this is not sufficient because an implosion could scatter hot flammable liquid. Use an explosion shield and a full-face shield to protect laboratory personnel, and carry the procedure out in a laboratory chemical hood. Glassware under vacuum should be kept behind a shield or hood sash, taped, or resin (plastic) coated.
Equipment at reduced pressure is especially prone to rapid pressure changes, which can create large pressure differences within the apparatus. Such conditions can push liquids into unwanted locations, sometimes with undesirable consequences.
Do not allow water, solvents, and corrosive gases to be drawn into a building vacuum system. When the potential for such a problem exists, use a cold trap. Water aspirators are not recommended.
Precautions to be taken when working with vacuum lines and other glassware used at sub ambient pressure are mainly concerned with the substantial danger of injury in the event of glass breakage. The degree of hazard does not depend significantly on the magnitude of the vacuum because the external pressure leading to implosion is always 1 atmosphere. Thus, evacuated systems using aspirators merit as much respect as high-vacuum systems. Injury due to flying glass is not the only hazard in vacuum work. Additional dangers can result from the possible toxicity of the chemicals contained in the vacuum system, as well as from fire following breakage of a flask (e.g., of a solvent stored over sodium or potassium).
Because vacuum lines typically require cold traps (generally liquid nitrogen) between the pumps and the vacuum line, precautions regarding the use of cryogens should be observed also. Liquid nitrogen–cooled traps open to the atmosphere condense liquid air rapidly. When the coolant is removed, an explosive pressure buildup occurs, usually with enough force to shatter glass equipment if the system has been closed. Hence, only sealed or evacuated equipment should be so cooled. Vacuum traps must not be left under static vacuum; liquid nitrogen in Dewar flasks must be removed from these traps when the vacuum pumps are turned off.
Residues from vacuum distillations have been known to explode when the still was vented suddenly to the air before the residue was cool. To avoid such explosions, vent the still pot with nitrogen, cool it before venting, or restore pressure slowly. Sudden venting may produce a shock wave that explodes sensitive materials.
Vacuum Pumps
Distillation or similar operations requiring a vacuum must use a trapping device to protect the vacuum source, personnel, and the environment. This requirement also applies to oil-free Teflon-lined diaphragm pumps. Normally the vacuum source is a cold trap cooled with dry ice or liquid nitrogen. Even with the use of a trap, the oil in a mechanical vacuum trap can become contaminated and the waste oil must be treated as a hazardous waste.
Vent the output of each vacuum pump to a proper air exhaust system. Vent lines may be Tygon, rubber or copper. If Tygon or rubber lines are used, they should be supported so that they do not sag or cause a trap for condensed liquids. Safe venting is essential when the pump is being used to evacuate a system containing a volatile toxic or corrosive substance. Failure to observe this precaution results in pumping volatile substances into the laboratory atmosphere.
Oil lubricated vacuum pumps should carry tags indicating the date of the most recent oil change. Vacuum pump oil should be changed once a month, or sooner if it is known that the oil has been unintentionally exposed to reactive gases. It may be desirable to maintain a log of pump usage as a guide to length of use and potential contaminants in the pump oil.
Belt-driven mechanical pumps must have protective guards. Such guards are particularly important for pumps installed on portable carts or tops of benches where laboratory personnel might accidentally entangle clothing,hair,or fingers in the moving belt or wheels.
Glass Vessels
Although glass vessels are frequently used in low-vacuum operations, evacuated glass vessels may collapse violently, either spontaneously from strain or from an accidental blow. Therefore, conduct pressure and vacuum operations in glass vessels behind adequate shielding. Check for flaws such as star cracks, scratches, and etching marks each time a vacuum apparatus is used. These flaws can often be noticed if the vessel is help up to a light. Use only round-bottom or thick-walled (e.g., Pyrex) evacuated reaction vessels specifically designed for operations at reduced pressure. Do not use glass vessels with angled or squared edges in vacuum applications unless specifically designed for the purpose (e.g., extra thick glass). Repaired glassware must be properly annealed and inspected with a cross-polarizer before vacuum or thermal stress is applied. Never evacuate thin-walled, Erlenmeyer, or round-bottom flasks larger than 1 liter.
Dewar Flasks
Glass dewar flasks are under high vacuum and can collapse as a result of thermal shock or a very slight mechanical shock. Shield them, either by a layer of fiber-reinforced friction tape or by enclosure in a wooden or metal container, to reduce the risk of flying glass in case of collapse. Use metal jacketed Dewar flasks whenever there is a possibility of breakage.
Styrofoam buckets with lids can be a safer form of short-term storage and conveyance of cryogenic liquids than glass vacuum Dewar flasks. Although they do not insulate as well as Dewar flasks, they eliminate the danger of implosion.
Assembly of Vacuum Apparatus
Assemble vacuum apparatus to avoid strain. Joints must allow various sections of the apparatus to be moved if necessary, without transmitting strain to the necks of the flasks. Support heavy apparatus from below as well as by the neck. Use a bubbler to protect vacuum and Schlenk lines from overpressure. Gas regulators and metal pressure-relief devices must not be relied on to protect vacuum and Schlenk lines from overpressure. If a slight positive pressure of gas on these lines is desired, the recommended pressure range is not in excess of 1 to 2 psi. This pressure range is easily obtained by proper bubbler design (depth of the exit tubing in the bubbler liquid).
Place vacuum apparatus well back onto the bench or into the laboratory chemical hood where it will not be inadvertently hit. If the back of the vacuum setup faces the open laboratory, protect it with panels of suitably heavy transparent plastic to prevent injury to nearby personnel from flying glass in case of implosion.
5.5.4 Drying Ovens and Furnaces
Volatile organics shall not be dried in ovens that vent to the room air. Glassware rinsed with organics should not be oven dried unless it is first re-rinsed with water. Bimetallic strip thermometers rather than mercury thermometers are recommended for measuring oven temperatures. If a mercury thermometer breaks in an oven, the oven shall be turned off and cooled before cleanup is attempted. Wear heat-resistant gloves and appropriate eye protection when working at ovens or furnaces. ANSI-approved eyewear (i.e., heat-absorbing, reflective goggles) offers protection against projectiles and infrared radiation.
5.5.5 Syringes and Scalpel Blades
Syringes used with hazardous agents shall have needle-locking or equivalent tips to assure that the needles cannot separate during use. Do not recap disposable needles after use. Recapping of needles potentially contaminated with human blood, blood products, or other potentially infectious materials is prohibited.
Syringes, needles, or scalpels shall be disposed of immediately after use in sealable, puncture-resistant, disposable containers that are leak-proof on the sides and bottom. The containers shall be appropriately labeled as to the chemical or biological hazard. Sharps containers shall be easily accessible to personnel in the immediate area of use.
5.5.6 Glassware and Plastic Labware
Borosilicate glassware, such as Pyrex 7740, is the type preferred for laboratory experimentation, except in special experiments involving ultraviolet or other light sources or hydrofluoric acid, for which polypropylene containers are most appropriate. Measuring glassware, stirring rods, tubing, and reagent bottles may be ordinary soft glass. Vacuum or suction flasks shall be designed with heavy walls. Dewar flasks and large vacuum vessels shall be taped or otherwise screened or contained in metal to prevent glass from flying if they should implode. An ordinary thin-walled thermos bottle is not an acceptable replacement for a Dewar flask.
Because glassware can be damaged in shipping, handling, or storage, inspect it carefully before using it to be sure it does not have hairline cracks or chips. Even the smallest flaw renders glassware unacceptable and possibly dangerous. Flawed glassware shall be discarded in a rigid, puncture-resistant broken-glass bin. Where the integrity of glassware is especially important, examine for strains using polarized light.
9Do not store strong oxidizing agents in plastic labware except that made of Teflon. Prolonged oxidizer exposure causes plastic embrittlement and failure.
5.5.7 Eliminating Mercury Thermometers and Mercury Containing Devices
Metallic mercury is highly toxic by skin absorption, inhalation, and ingestion. Lab members face limited potential exposure whenever they break mercury-filled thermometers. The mercury contamination may infiltrate cracks in benches and the floor or spread beneath equipment and instruments. The contamination is insidious and difficult to remove completely. The difficulty is magnified if the thermometer breaks in a water bath or sink.
One of the best methods for eliminating this hazard and metallic mercury in laboratory spaces is to replace all mercury thermometers with non-mercury instruments. Alternatives to mercury thermometers are spirit-filled or digital units. Research Safety strongly urges you to substitute non-mercury thermometers whenever possible.
Laboratories should pursue alternatives to other mercury-containing laboratory equipment—such as mercury bubblers—whenever feasible. Replacement of such equipment eliminates mercury-based spill and/or exposure hazards.
Alkyl mercury compounds are extremely toxic and require prior approval from Research Safety before purchase or use.
5.5.8 Ultraviolet, Visible, and Near-Infrared Radiation10
Ultraviolet, visible, and infrared radiation from lamps and lasers in the laboratory space can produce a number of hazards. Medium-pressure Hanovia 450 Hg lamps are commonly used for ultraviolet irradiation in photochemical experiments. Ultraviolet lights used in biosafety cabinets, as decontamination devices, or in light boxes to visualize DNA can cause serious skin and corneal burns. Powerful arc lamps can cause eye damage and blindness within seconds. Some compounds (e.g., chlorine dioxide) are explosively photosensitive.
When incorrectly used, the light from lasers poses a hazard to the eyes of the operators and other people present in the room and is also a potential fire hazard. See the Laser Safety Handbook for further details about laser registration and hazard control. Glassblowing and the use of laser or ultraviolet light sources require special eye protective glasses or goggles.
5.5.9 Magnetic Fields
See Magnetic Fields on the Research Safety website.
5.5.10 Radio Frequency and Microwave Hazards
Radio frequency (rf) and microwaves occur within the range 10 kHz to 300,000 MHz and are used in rf ovens and furnaces, induction heaters, and microwave ovens. Extreme overexposure to microwaves can result in the development of cataracts or sterility or both. Microwave ovens may be used in laboratory spaces for organic synthesis and digestion of analytical samples. Only microwave ovens designed for laboratory or industrial use should be used in a laboratory. Use of metal in microwave ovens can result in arcing and, if a flammable solvent is present, in fire or explosion. Superheating of liquids can occur. Capping of vials and other containers used in the oven can result in explosion from pressure buildup within the vial. Inappropriately selected plastic containers may melt.11
Microwave heating presents several potential hazards not commonly encountered with other heating methods: extremely rapid temperature and pressure rise, liquid superheating, arcing, and microwave leakage. Microwave ovens designed for the laboratory have built-in safety features and operation procedures to mitigate or eliminate these hazards. Users of such equipment must be thoroughly knowledgeable of operation procedures and safety devices and protocols before beginning experiments, especially when there is a possibility of fire (flammable solvents), over-pressurization, or arcing (Foster and Cournoyer, 2005).
To avoid exposure to microwaves, never operate ovens in disrepair. Do not place wires and other objects between the sealing surface and the door on the oven’s front face. Keep the sealing surfaces absolutely clean. To avoid electrical hazards, the oven must be grounded. If use of an extension cord is necessary, use only a three-wire cord with a rating equal to or greater than that for the oven. To reduce the risk of fire in the oven, do not overheat samples. The oven must be closely watched when combustible materials are in it. Do not use metal containers or metal-containing objects (e.g., stir bars) in the microwave, because they can cause arcing.
In general, do not use heat sealed containers in a microwave oven, because of the danger of explosion. If sealed containers must be used, select their materials carefully and the containers properly designed. Commercially available microwave acid digestion bombs, for example, incorporate a Teflon sample cup, a self-sealing Teflon O-ring, and a compressible pressure-relief valve. Do not exceed the manufacturer’s loading limits. For such applications, properly vent the microwave oven using an exhaust system. Placing a large item, such as a laboratory microwave or an oven, inside a chemical fume hood is not recommended.
Heating a container with a loosened cap or lid poses a significant risk. Microwave ovens can heat material (e.g., solidified agar) so quickly that, even though the container lid is loosened to accommodate expansion, the lid can seat upward against the threads and the container can explode. Screw caps must be removed from containers being microwaved. If the sterility of the contents must be preserved, screw caps may be replaced with cotton or foam plugs.
Although industrial ovens may reduce the risk of such hazards, significant caution is required in their use. In general, the use of closed vessels should be avoided. Any reactions conducted in a microwave oven should be regarded with the same caution as those conducted with highly reactive and explosive chemicals. Reactions should use the smallest scale possible to determine the potential for explosions and fires. Precautions should be taken for proper ventilation and potential explosion.12
5.5.11 Extreme Noise
Any laboratory operation that exposes trained laboratory personnel to a significant noise source of 85 decibels(A) or greater for an 8-hour average duration should have a hearing conservation program to protect from excessive exposure. Consult Research Safety to determine the need for such a program and to aid in developing one.
Ultra-sonicators may expose lab members to inaudible mechanical frequencies between 16 and 100 kHz. Resulting subharmonic frequencies may be audible. Airborne conduction, direct contact through a liquid-coupling medium, and direct contact with a vibrating solid are routes of exposure. Avoid direct body contact with liquids or solids subjected to a high-intensity ultrasonic source. Low intensity ultrasound is safely used for imaging of the human body.
5.6 Facility Cleaning and Maintenance
A custodial service is contracted to wet-mop floors (including laboratory space) regularly. However, building services and custodial staff are prohibited from cleaning up chemical and biological materials (including spills), and custodians shall not be expected to mop any floors that have not been properly decontaminated after a spill.
In preparation for the cleaning service, the laboratory staff shall remove hazards that the custodians might encounter during their activities. Laboratory occupants shall move chemical and biohazardous waste containers off the floor to a safe and secure location before custodians perform floor cleaning. In the event that a laboratory supervisor does not wish a particular laboratory space to be disturbed, custodial floor cleaning can be suspended. To have the floor cleaning discontinued, post a sign on the lab door. Signs are available from the Research Safety office.

Likewise, if maintenance is required on any component of the laboratory, such as a sink or piece of equipment, the same principles of preparation apply. The supervisor shall ensure that the immediate area is decontaminated and any infectious agents or chemicals are removed to another secure area prior to initiation of work. The laboratory supervisor shall inform maintenance personnel of the presence of any hazardous materials to which they might become exposed. Facility maintenance and custodial staff shall not handle or remove hazardous waste bags or other containers.
Cleaning duties that are the specific responsibility of laboratory personnel shall be conducted on a regular basis to prevent accidental contact with hazards and to reduce clutter in the laboratory space. Laboratory equipment, including refrigerators, freezers, and work surfaces, shall be cleaned by laboratory staff. In laboratories using large amounts of powdered carcinogens, reproductive toxins, or acutely toxic materials, lab members should avoid dry mopping or sweeping with a broom if this could cause the materials to become airborne. Pregnant women shall not be assigned to clean hazardous materials spills.
Facility maintenance and custodial staff shall not handle or remove hazardous waste bags or other containers.
5.7 Signs and Labels for Laboratories
An “Emergency Information” sign shall be posted outside all laboratories, either on the outside of the door or on the wall beside the door. This sign shall provide information on special precautions for entry, and telephone numbers of responsible faculty and staff.
5.8 Laboratory Safety and Chemical Hygiene Training
Laboratory member safety training is required under the OSHA Laboratory Standard and additional general industry standards (e.g., the OSHA Personal Protective Equipment Standards, Respiratory Protection Standard, etc.). University policy prohibits persons without appropriate training from being assigned to work independently in laboratory spaces and other areas where hazardous chemicals are used.
Research Safety provides general, regulatory-required laboratory safety training to laboratory members in order to begin their training process. PIs shall provide personalized, hands-on training that ensures lab members are familiar with safe work practices, engineering controls, and personal protective equipment required to safely conduct the hazardous processes, operate equipment or machinery, and handle chemicals specific to their laboratory.
PIs can manage training requirements for Research Safety-provided training inside the Training Module of Lumen. Lab members can use the Training Module to view their training status and enroll in safety training courses.
Research Safety is available to assist with evaluation of training requirements and can provide general safety seminars for laboratory or department groups.
6.0 Chemical Hazards
“What is it that is not poison? All things are poison, and nothing is without poison. It is the dose only that makes a thing not a poison.” — Paracelsus (1493 – 1541)
6.1 Hazard Communication
The United States has adopted the United Nations Globally Harmonized System of Classification and Labeling of Chemicals (GHS). The GHS is a comprehensive approach to defining a chemical’s hazards and communicating those hazards and protective measures to members.
Pictograms identify health, physical and environmental hazards associated with a chemical.
Table 6.1
| PICTOGRAM | HAZARD CLASSIFICATION |
|---|---|
|
|
Gases Under Pressure |
 |
Environmental Toxicity |
 |
Acute Toxicity (Severe) |
|
|
Corrosives |
|
|
Oxidizers |
 |
Carcinogen Respiratory Sensitizer Reproductive Toxicity Target Organ Toxicity Mutagenicity Aspiration Toxicity |
|
|
Explosives Self-reactives Organic Peroxides |
 |
Irritant Dermal Sensitizer Acute Toxicity (harmful) Narcotic Effects Respiratory Tract Irritation |
|
|
Flammables Self-reactives Pyrophorics Self-heating Emits Flammable Gas Organic Peroxides |
Each hazard classification contains one or more hazard categories indicating the degree of the hazard, with Category 1 being the most hazardous.
6.1.1 Container Labels and Material Safety Data Sheets (MSDSs)
MSDSs are the most basic source of chemical hazard information. The MSDS summarizes the chemical’s properties, the health and physical hazards, including the type of toxicity information discussed in the sections below, and related safety information required by emergency responders.
6.1.2 Dating Containers
Chemical containers shall be dated on receipt in the laboratory space and on opening. This information provides a history of the chemicals in each container and guides future researchers as to potential quality of the chemicals stored in the laboratory space. Providing container-opening dates is especially important for peroxide-forming chemicals sold or distilled without added autoxidation inhibitors. Solutions shall be labeled and dated when prepared and after each test for peroxides. Chemicals shall be removed from the laboratory space if they are past their expiration date.
6.2 Exposure to Chemicals
The complex relationship between a material and its biological effect in humans involves considerations of dose, duration and frequency of the exposure, route of exposure, and many other factors, including sex, allergic factors, age, previous sensitization, and lifestyle.
The risk management guidelines from the American Chemical Society13 are organized around the concept of RAMP, an acronym for the four principles of safety:
- Recognize the hazard
- Assess the risk
- Minimize the risk
- Prepare for emergencies
6.2.1 Exposure Routes
Chemicals enter the body through the following routes:
- Inhalation — absorption through the respiratory tract by inhalation is the easiest way for chemicals to enter the body.
- Ingestion — absorption through the digestive tract by eating or smoking with contaminated hands or in contaminated work areas. Depending on particle or droplet size, aerosols may also be ingested.
- Skin or eye contact — absorption through the skin or eyes. Skin contact is the most common cause of the widespread occupational disease dermatitis. The eyes are very porous and can easily absorb toxic vapors that cause permanent eye damage.
- Injection — percutaneous injection through the skin. This can occur through misuse of sharp items, especially hypodermic needles.
Toxic effects can be immediate or delayed, reversible or irreversible, local or systemic.
6.2.2 Acute and Chronic Toxicity
A thorough discussion of toxicity is beyond the scope of any single publication. Information is available in Safety Data Sheets (SDSs) and other reference materials that are available at Research Safety on each campus. Toxicity is the measure of a poisonous material’s adverse effect on the human body or its ability to damage or interfere with the metabolism of living tissue. Generally, toxicity is divided into two types, acute and chronic. Many chemicals may cause both types of toxicity, depending on the pattern of use.
Acute toxicity is an adverse effect with symptoms of high severity coming quickly to a crisis. Acute effects are normally the result of short-term exposures and are of short duration. Examples of acutely toxic chemicals are hydrogen cyanide and ammonia.
Chronic toxicity is an adverse effect with symptoms that develop slowly over a long period of time as a result of frequent exposure. The dose during each exposure period may frequently be small enough that no effects are noticed at the time of exposure. Chronic effects are the result of long-term exposure and are of long duration, for example exposure to carcinogens as well as many metals and their derivatives. Cumulative poisons are chemicals that tend to build up in the body as a result of numerous chronic exposures, leading to chronic toxicity. The effects are not seen until a critical body burden is reached. Examples of cumulative poisons are lead and mercury.
With substances in combination, such as exposure to two or more hazardous materials at the same time, the resulting effect can be greater than the combined effect of the individual substances. This is called a synergistic or potentiating effect. One example is concurrent exposure to alcohol and chlorinated solvents.
The published toxicity information for a given substance is general—human data may not be available—and the actual effects can vary greatly from one person to another. Do not underestimate the risk of toxicity. All substances of unknown toxicity should be handled as if they are toxic, with the understanding that any mixture may be more toxic than its most toxic component.
Solvent Exposure Chart
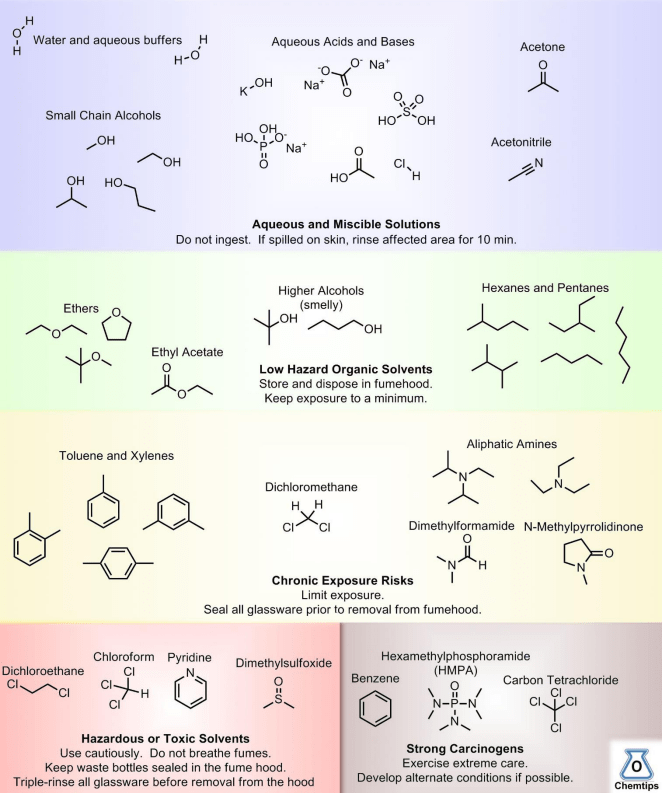
6.2.3 Carcinogenicity
A carcinogen is a chemical that causes malignant (cancerous) tumors.
See the International Agency for Research on Cancer (IARC) for more information.
6.2.4 Reproductive Toxins
Chemicals can affect both adult male and female reproductive systems. Chemicals may also affect a developing fertilized ovum, embryo, or fetus through maternal exposure (teratogenic effects). Reproductive hazards affect people in a number of ways, including mental disorders, loss of sexual drive, impotence, infertility, sterility, mutagenic effects on cells, teratogenic effects on the fetus, and transplacental carcinogenesis. Exposure to lead or to anticancer drugs, such as methotrexate, or to ionizing radiation can cause infertility, miscarriage, birth defects, and low birth weight. Certain ethylene glycol ethers such as 2-ethoxyethanol and 2-methoxyethanol can cause miscarriages. Carbon disulfide can cause menstrual cycle changes. One cannot assume that any given substance is safe if no data on gestational exposure are available.
Specific hazards of chemical exposure are associated with the male reproductive system, including suppression of sperm production and survival, alteration in sperm shape and motility, and changes in sexual drive and performance. Various reproductive hazards have been noted in males following exposure to halogenated hydrocarbons, nitro aromatics, arylamines, ethylene glycol derivatives, mercury, bromine, carbon disulfide, and other chemical reagents.
Consult the SDS for information on known reproductive hazards. The following are additional resources on reproductive toxins and carginogens:
- List of Reproductive Toxins
- National Institute for Occupational Safety and Health (NIOSH)
6.3 Designated Area
Work involving selected carcinogens, reproductive toxins, and substances of high acute toxicity shall be conducted in a “designated area.” This is a requirement of the OSHA Laboratory Standard. This area shall be so posted, and all employees working within the area shall be informed of the hazardous substances used there. The designated area may be a chemical fume hood, a part of a laboratory, or the entire laboratory space.
6.4 Monitoring Airborne Concentrations of Contaminants
OSHA has established permissible exposure limits (PELs) for airborne concentrations of selected materials. The PEL is defined as a time-weighted average (TWA) concentration of a particular substance for a normal 8-hour workday and a 40-hour workweek, a concentration to which nearly all workers may be exposed, day after day, without adverse effect.
Corollaries to the 8-hour PEL are the short-term exposure limit (STEL) and the ceiling exposure limit. The STEL is the time-weighted average concentration of a compound to which a worker may be exposed over a period of 15 minutes without expecting symptoms of irritation, chronic or irreversible tissue damage, or narcosis. The ceiling is the concentration of a substance that should not be exceeded during any part of the working exposure. When instantaneous monitoring is not feasible, the ceiling limit is measured over a period of 10 to 15 minutes.
As the PELs were designed to protect workers in industrial settings, it is unlikely that these limits will be exceeded during the performance of laboratory procedures. Laboratory workers generally do not handle the same quantities of hazardous materials as do manufacturing and production employees.
Nonetheless, exposure to airborne chemicals shall not exceed PELs. If there is reason to believe that airborne concentrations may exceed PELs, contact Research Safety for consultation on the need for air monitoring. PELs are listed on Safety Data Sheets, or may be found on the OSHA PEL web page. Please note that PELs have not been developed for all the compounds to which laboratory workers may be exposed. In all circumstances, caution shall be used in handling hazardous chemicals.
In addition to PELs, OSHA has set action levels for specific compounds, such as formaldehyde, cadmium, and lead, for which individual standards have been promulgated. OSHA has classified these compounds as potential carcinogens.
If monitoring of airborne concentrations reveals that levels are above the OSHA action level, then levels shall either be immediately reduced by a procedural change or equipment modification or the department head and PI shall comply with the requirements of the OSHA standard for the chemical. OSHA regulations govern periodic monitoring and termination of monitoring, as well as employee notification. Medical surveillance may be a requirement.
For chemicals without regulated action levels, the general rule is that half the PEL may be considered a de facto action level. Engineering controls shall be instituted to reduce exposure to the hazardous substance in question.
6.5 Guidelines for Handling Chemicals
The chemical handling guidelines described in this document are founded on several basic principles:
- Substitute less hazardous chemicals whenever possible.
- Minimize chemical exposures.
- Avoid underestimating risk especially when scaling up.
- Provide adequate ventilation.
Since many chemicals used in research are hazardous to some degree, it is prudent to minimize exposure to research chemicals as a general rule, rather than implementing safety protocols only for specific compounds. Avoid skin contact with chemicals as much as possible. Assume that mixtures are more toxic than their components and that all substances of unknown toxicity are toxic. Do not work with a volatile or aerosolizing material without adequate ventilation from chemical fume hoods or other protective devices. Remember: Wise up, suit up, clean up!
6.5.1 General Guidelines
The following guidelines are applicable to nearly all uses of chemicals in laboratory spaces. They apply to most hazardous chemicals, such as acids, bases, and flammable liquids. They are also applicable to chemicals that display low carcinogenic potency in animals and are not considered carcinogens.
The general guidelines are not, by themselves, adequate for chemicals with high acute toxicity or high chronic toxicity such as heavy metals, chemical carcinogens, or reproductive toxins.
- Wear eye protection at all times where chemicals are used or stored.
- Wear a lab coat or other protective clothing (e.g., apron).
- Wear gloves selected on the basis of the hazard. Inspect them before use. Wash reusable gloves before removal. Turn disposable gloves inside out carefully when removing to avoid contaminating hands.
- Wash hands immediately after removing gloves, after handling chemical agents, and before leaving the laboratory space.
- Lab coats shall be cleaned frequently.
- Confine long hair and loose clothing.
- Wear sturdy shoes that cover feet completely.
- Do not store or prepare food, eat, drink, chew gum, apply lip balm or cosmetics, or handle contact lenses in areas where hazardous chemicals are present.
- Food is stored in cabinets or refrigerators designated for such use only.
- Never pipette or start a siphon by mouth.
- Label all chemical containers.
- Chemical storage is by hazard class. Chemicals are not stored merely by alphabetical order, carbon count or functional groups.
- Never sniff or taste chemicals. Again, label containers properly to avoid confusion about contents.
- Keep work areas clean and uncluttered.
- Keep personal belongings away from chemicals.
- Obtain an SDS for each chemical, and consult the SDS before you use a chemical.
- Know the emergency procedures for the building, the department, and the chemicals being used.
- Vent into local exhaust devices any apparatus that may discharge toxic vapors, fumes, mists, dusts, or gases. Never release toxic chemicals into cold rooms or warm rooms that have recirculating atmospheres.
- In cold rooms, to prevent mold growth conditions from releasing toxins, replace all storage cardboard boxes with plastic containers. Research Safety has plastic trays available for this purpose.
- Use chemical fume hoods or other engineering controls to minimize exposure to airborne contaminants.
- Properly handle, collect, and dispose of surplus and waste chemicals.
6.5.2 Guidelines for Working with Chemicals of Acute Toxicity
Chemicals of acute toxicity are defined by OSHA as those that cause rapid effects as a result of a short-term exposure—generally sudden and severe, as in the case of a leak from equipment. Acute toxic effects include irritation, corrosion, sensitization, and narcosis.
Alkyl mercury compounds require prior approval from Research Safety before purchase or use.
To illustrate, hydrofluoric acid (HF) is a chemical of high acute toxicity because of its destructive effect on skin and bone tissue. Arsine and other hydrides may be lethal at low concentrations because of red blood cell hemolysis. Inhalation of high concentrations of carbon monoxide can cause immediate poisoning and possible death, as the gas directly interferes with oxygen transport in the body by preferentially binding to hemoglobin. Hydrogen cyanide inhalation inhibits enzyme systems vital to cellular uptake of oxygen.
When working with significant quantities of such chemicals, the aim is to minimize exposure to the material. Special care should be taken in the selection of protective clothing to ensure it is appropriate for the hazard. Personal hygiene and work practices should also be carefully evaluated to minimize exposure. The following guidelines should be practiced in addition to the general guidelines for handling chemicals.
- When performing procedures that may result in the release of airborne contaminants, use a chemical fume hood.
- Trap or treat effluents to remove gases, fumes, vapors, and particulates before discharging them to facility exhaust.
- Restrict access to the laboratory or work area.
- Establish and label a “designated area” for work with acutely toxic chemicals. Keep materials within the designated area.
- Use plastic-backed paper or trays under work areas. Replace the paper when contaminated.
- Develop and know special emergency procedures.
- Keep emergency supplies at hand for immediate use. When hydrofluoric acid is in use, the first aid kit must contain calcium gluconate gel.
6.5.3 Guidelines for Chemicals with High Chronic Toxicity, Carcinogens, and Reproductive Toxins
In addition to the general guidelines for handling chemicals, use the following guidelines for handling chemicals with high chronic toxicity, which include most heavy metals, chemicals displaying moderate to high carcinogenic potency in animals, and reproductive toxins.
- For carcinogens, determine if the chemical is regulated by OSHA in a substancespecific standard. If so, the PI or lab supervisor shall document a hazard evaluationin the Lumen Profile.
- Designated work and storage areas shall be established for carcinogens, chemicals with high chronic toxicity, and reproductive toxins. Materials shall be kept within the designated area to the extent possible.
- Designated work and storage areas for chemical carcinogens, including chemical fume hoods and refrigerators, shall be labeled “Chemical Carcinogen. Designated work and storage areas used for chemicals with high chronic toxicity or reproductive toxins shall be labeled “Toxic Chemical” or “Toxic Substance.”
- Access procedures shall be used if work involves moderate or greater amounts of carcinogens or moderate to lengthy procedures. These procedures may include:
- Closed doors
- Restricted access—only authorized personnel permitted
- Written access procedures posted on the outer door.
- Cover laboratory surfaces, including chemical fume hood surfaces, with plastic-backed paper or protective trays. Inspect work surfaces following procedures, and remove the paper if contamination is present. Dispose of the used paper as hazardous waste.
- Disposable gloves shall be disposed of as hazardous waste. Wash reusable gloves before removing them. Contact Research Safety prior to washing to determine if the wash water must be collected for disposal as a hazardous waste.
- Transport highly toxic or carcinogenic materials through public areas, such as hallways, in closed containers within unbreakable outer containers. Sealed plastic bags may be used as secondary containment in many cases.
- To avoid potential inhalation hazards, handle powdered carcinogens and toxins in a chemical fume hood, even during weighing procedures. Inside the chemical fume hood, measure the powder with a spatula into a pre-weighed vessel then seal or cover the vessel, remove it from the chemical fume hood, and take it to the balance to be weighed. If more or less material is needed, return the container to the chemical fume hood for addition or subtraction of material. Close the container again and reweigh it. Repeat these steps until the desired amount is obtained. This procedure eliminates contamination of the air, the work bench, and the scale. Procedures generating either solid or liquid airborne contaminants or involving volatile chemicals are always to be performed in a chemical fume hood.
- Vacuum pumps shall be protected against contamination (e.g., traps and filters in lines) and vented into direct exhaust ventilation. Pumps and other equipment and glassware shall be decontaminated before they are removed from the designated area. The designated area shall be decontaminated before other normal work is conducted. Vacuum pump oil shall be collected as a contaminated waste and disposed of through Research Safety.
- Water vacuum lines shall be equipped with traps to prevent vapors from entering the wastewater stream.
- Floors shall be wet-mopped or cleaned with a high-efficiency particulate air filter (HEPA) vacuum cleaner if powdered materials are used.
6.6 Chemical Emergency Procedures
6.6.1 Procedures for Spills of Volatile, Toxic, or Flammable Materials
- Warn all persons nearby.
- Turn off any ignition sources such as burners, motors, and other spark-producing equipment.
- Leave the room and close the door if possible.
- Call University Police at 456 to report the hazardous material spill. University Police will contact Research Safety emergency response personnel at any time to respond to hazardous material spills.
Small spills can be absorbed with paper towels or other absorbents. However, these materials can increase the surface area and evaporation rate, increasing the potential fire hazard if the material is flammable and airborne concentration reaches the flammability level.
Pregnant women shall not be assigned to clean hazardous materials spills.
6.6.2 Procedures for Chemical Spill on a Person
- Know where the nearest eyewash and safety shower are located.
- For small spills on the skin, flush immediately under running water for at least 15 minutes, removing any jewelry that might contain residue. If there is no sign of a burn, wash the area with soap under warm running water. If pain returns after the 15-minute flooding, resume flooding the area. When providing assistance to a victim of chemical contamination, use appropriate personal protective equipment.
- See also Section 5.1.1 First Aid Procedure for Responding to Hydrofluoric Acid Burns.
- For a chemical splash in the eyes, immediately flush the eyes under running potable water for 15 minutes, holding the eyes open and rotating the eyeballs. This is preferably done at an eyewash fountain with tepid water and properly controlled flow. Hold the eyelids open and move the eye up, down, and sideways to ensure complete coverage. If no eyewash fountain is available, put the victim on his or her back and gently pour water into the eyes for 15 minutes or until medical personnel arrive.
- For spills on clothing, immediately remove contaminated clothing, including shoes and jewelry, while standing under running water or the safety shower. When removing shirts or pullover sweaters, be careful not to contaminate the eyes. Cutting off such clothing will help prevent spreading the contamination. To prepare for emergencies, shears (rounded-tip scissors) should be available in the first aid kit to allow safe cutting of contaminated clothing.
- Consult the SDS to see if any delayed effects should be expected and keep the SDS with the victim. Call UP to have the victim taken to the emergency room for medical attention. Be sure to inform emergency personnel of the decontamination procedures used prior to their arrival (for example, flushing for 15 minutes with water). Be certain that emergency room personnel are told exactly what the victim was contaminated with so they can treat the victim accordingly.
6.6.3 Procedure for Cryogenic Liquid Spill on a Person
Contact with cryogenic liquids may cause crystals to form in tissues under the spill area, either superficially or more deeply in the fluids and underlying soft tissues. The first aid procedure for contact with cryogenic liquids is identical to that for frostbite. Re-warm the affected area as quickly as possible by immersing it in warm, but not hot, water (between 102° and 105° F). Do not rub the affected tissues. Do not apply heat lamps or hot water and do not break blisters. Cover the affected area with a sterile covering and seek assistance as you would for burns.
6.6.4 Incidental Spills–Procedure for Small, Low-Toxicity Chemical Spills
Be prepared. Keep appropriate spill-containment material on hand for emergencies. Consult with Research Safety to determine which materials are suitable in a particular laboratory space.
Laboratory members must receive training to distinguish between the types of spills they can handle on their own and those spills that are classified as “MAJOR.” Major spills dictate the need for outside help.
Laboratory members are qualified to clean-up spills that are “incidental.” OSHA defines an incidental spill as a spill that does not pose a significant safety or health hazard to employees in the immediate vicinity nor does it have the potential to become an emergency within a short time frame. The period that constitutes a short time is not defined. Laboratory members can handle incidental spills because they are expected to be familiar with the hazards of the chemicals they routinely handle during an “average” workday. If the spill exceeds the scope of the laboratory workers’ experience, training or willingness to respond, the workers must be able to determine that the spill cannot be dealt with internally.
Emergency assistance is provided by Research Safety or an outside agency. Spills requiring the involvement of individuals outside the laboratory space are those exceeding the exposure one would expect during the normal course of work. Spills in this category are emergency situations. Responders need additional training and personal protective equipment.
Factors that clearly indicate a major spill are:
- The need to evacuate employees in the area
- The need for response from outside the immediate release area
- The release poses, or has potential to pose, conditions that are immediately dangerous to life and health
- The release poses a serious threat of fire and explosion
- The release requires immediate attention due to imminent danger
- The release may cause high levels of exposure to toxic substances
- There is uncertainty that the member can handle the severity of the hazard with the PPE and equipment that has been provided and the exposure limit could be easily exceeded, and
- The situation is unclear or data is lacking regarding important factors.
The following steps shall be followed for incidental spills:
- Alert persons in the area that a spill has occurred.
- Evaluate the toxicity, flammability, and other hazardous properties of the chemical as well as the size and location of the spill (for example, chemical fume hood or elevator) to determine whether evacuation or additional assistance is necessary. Large or toxic spills are beyond the scope of this procedure.
- Contain any volatile material within a room by keeping doors closed. Increase exhaust efficiency by minimizing sash height of the chemical fume hood or activating the emergency purge, if available.
- Consult your SDS, the laboratory emergency plan, or procedures in this document, or call Research Safety for correct cleaning procedures.
- Obtain cleaning equipment and protective gear from Research Safety, if needed.
- Wear protective equipment such as goggles, apron, laboratory coat, gloves, shoe covers, or respirator. Base the selection of the equipment on the hazard.
- First cordon off the spill area to prevent inadvertently spreading the contamination over a much larger area.
- Absorb liquid spills using paper towels, spill pillows, vermiculite, or sand. Place the spill pillow over the spill and draw the free liquid into the pillow. Sprinkle vermiculite or sand over the surface of the free liquid.
- Place the used pillows or absorbent materials in plastic bags for disposal along with contaminated disposable gear, such as gloves.
- Neutralize spills of corrosives and absorb, if appropriate. Sweep up waste and place in plastic bags for disposal.
- Complete a Hazardous Waste Pickup Request. Research Safety will pick up the wastes.
- Complete an Incident Report Form describing the spill and send a copy to Research Safety. A copy may be kept by the department head, if required.
Note: Consult the SDS.
TABLE 6.6.4 QUICK REFERENCE FOR SPILL CLEANUPS14
| CHEMICAL SPILLED | CLEANUP |
|---|---|
| Acids, organic | Apply sodium bicarbonate. Absorb with spill pillow or vermiculite. |
| Acids, inorganic | Apply sodium bicarbonate/calcium oxide or sodium carbonate/calcium oxide. Absorb with spill pillow or vermiculite. Note: Hydrofluoric acid is an exception to this general practice; see below. |
| Acid chlorides | Do not use water. Absorb with sand or sodium bicarbonate. |
| Aldehydes | Absorb with spill pillow or vermiculite. |
| Aliphatic amines | Apply sodium bisulfite. Absorb with spill pillow or vermiculite. |
| Aromatic amines | Absorb with spill pillow or vermiculite. Avoid skin contact or inhalation. |
| Aromatic halogenated amines | Absorb with spill pillow or vermiculite. Avoid skin contact or inhalation. |
| Azides (potential explosives) | Absorb with spill pillow or vermiculite. Decontaminate with 10% ceric ammonium nitrate solution. |
| Bases (caustic alkalis) | Neutralize with acid or commercial chemical neutralizers and absorb with spill pillow or vermiculite. |
| Carbon disulfide (flammable and toxic) | Absorb with spill pillow or vermiculite. |
| Chlorohydrins | Absorb with spill pillow or vermiculite. Avoid skin contact or inhalation. |
| Cyanides | Wet or mist solids before sweeping, or use a HEPA filter vacuum to collect the solids. Absorb liquids with spill pillow or vermiculite. |
| Halides, organic or inorganic | Apply sodium bicarbonate. |
| Halogenated hydrocarbons | Absorb with spill pillow or vermiculite. |
| Hydrazine | Absorb with spill pillow or vermiculite. Avoid organic matter. |
| Hydrofluoric acid | Absorb with calcium carbonate (or calcium oxide) rather than sodium bicarbonate. The use of sodium bicarbonate will lead to the formation of sodium fluoride, which is considerably more toxic than calcium fluoride. Be careful in the choice of spill pillows used to absorb the acid. Certain pillows contain silicates that are incompatible with hydrofluoric acid. |
| Inorganic salt solutions | Apply soda ash. |
| Mercaptans/organic sulfides | Neutralize with calcium hypochlorite solution. Absorb with spill pillow or vermiculite. |
| Nitriles | Sweep up solids. Absorb liquids with spill pillow or vermiculite. |
| Nitro compounds, organic nitros | Absorb with spill pillow or vermiculite. Avoid skin contact or inhalation. |
| Oxidizing agents | Apply sodium bisulfite. |
| Peroxides | Absorb with spill pillow or vermiculite. |
| Phosphates, organic and related | Absorb with spill pillow or vermiculite. |
| Reducing substance | Apply soda ash or sodium bicarbonate. |
6.6.5 Mercury Spill Response Procedure
Use of mercury containing devices is discouraged. Thermometers, manometers and switches that contain mercury should be labeled. Mercury is a high-density, low-viscosity liquid at room temperature. During a spill, mercury can form tiny droplets that adhere to surfaces and enter cracks and crevices. Research Safety has a mercury vapor analyzer available to assist with large or difficult-to-clean mercury spills. In the case of small mercury spills (e.g., mercury-containing thermometers), laboratory personnel should be able to handle the cleanup. Cleanup kits are available from Research Safety.
To minimize the spill hazard, place a splash plate beneath all mercury-containing equipment.
Procedures for small mercury spills:
Equipment needed – Mercury Spill Kit from Research Safety
- Mercury vacuum pump, eyedropper, water or vacuum drive aspirator (optional)
- Chemical amalgam
- Laboratory coat
- Gloves
- Shoe protectors
- Glass or plastic collection container
- Plastic bags
- Wipes or paper towels
- Barricade tape
- Before entering the contaminated area, put on protective clothing.
- Establish a cleanup area and section it off to avoid spreading mercury.
- Draw all visible mercury into a glass or plastic collection container.
- Sprinkle the contaminated area with chemical amalgam. Wet with a little water.
- Wipe up the powder from the contaminated area with a wet towel or a damp sponge impregnated with chemical amalgam. Repeat steps 4 and 5.
- Sprinkle a very light coating of chemical amalgam into the cracks and crevices.
- Dispose of the contaminated solid waste material (such as boots, gloves, wipes, or thermometer glass) in a plastic bag and seal tightly.
- Dispose of the collected mercury and the bags of waste through Research Safety. Do not bring the waste bag to Research Safety; it will be picked up from your laboratory. Store the bag in a chemical fume hood until it is collected by Research Safety.
- The PI shall ensure that an Incident Report Form is completed and sent to Research Safety.
6.7 Medical Consultation or Surveillance
Medical surveillance is the systematic assessment of employees exposed or potentially exposed to occupational hazards. This assessment monitors individuals for adverse health effects and determines the effectiveness of exposure prevention strategies. A medical surveillance program includes the analysis of both individual and aggregate surveillance data over time, with the goal of reducing and ultimately preventing occupational illness and injury. Medical consultation or surveillance may be required whenever lab members experience sign or symptoms or expected or unexpected high exposure levels to hazardous chemicals.
6.7.1 Medical Consultation
Medical consultation is provided by a licensed health care provider without cost to the employee or student, without loss of pay, and at a reasonable time and place. For employees, medical consultation or examinations shall be provided through the Workers’ Compensation Program. For students, the medical program is administered through the University Health Service facilities.
The PI or laboratory supervisor shall ensure that the following information is provided to the physician: the identity of the chemical involved in the exposure, a description of conditions relating to the exposure, any quantitative data available regarding the exposure, and a description of signs and symptoms experienced by the affected person.
Medical consultation and examination is also required for lab members assigned to wear a respirator. See the University’s Respiratory Protection Program for additional information.
6.7.2 Medical Surveillance for Chemicals of High Chronic Toxicity
Routine medical surveillance may be warranted for individuals working with chemicals of high chronic toxicity, including carcinogens. Specific examples15 include , nickel carbonyl, benzo-a-pyrene, N-nitrosodiethylamine and allyl mercury compounds .
Candidates for work with carcinogens shall be informed of the possibility of increased risk. Job tasks for certain members using chemicals of high chronic toxicity should be evaluated to determine whether these members should be temporarily excluded from work or reassigned to less hazardous activities. This is particularly appropriate for pregnant women or persons receiving immunosuppressive drugs or therapy.
6.8 Chemical Storage
Practices that encourage the appropriate labeling and storage of chemicals can reduce the risks of mixing of incompatible chemicals and assist with regulatory compliance16.
Highly hazardous chemicals must be stored in a well-ventilated secure area that is designated for this purpose. Cyanides must be stored in a tightly closed container that is securely locked in a cool dry cabinet to which access is restricted. Protect cyanide containers against physical damage and separate them from incompatibles.
Flammable liquids should be stored in approved flammable-liquid containers and storage cabinets. Observe National Fire Protection Association, International Building Code, International Fire Code, and other local code requirements that limit the quantity of flammables per cabinet, laboratory space, and building.
In the Chicago campus research spaces, a single container capacity for NFPA Class I flammable liquids is limited to 4 liters. The Laboratory Unit Fire Hazard Class17 is limited to Class C – low fire hazard. This means that the maximum quantity of Class I flammable liquids, in fire rated storage cabinets, is limited to 15 liters per 9.3 m2 (100sf) of research space.
Store odiferous materials in ventilated cabinets. Use of corrosion-resistant storage trays as secondary containment for spills, leaks, drips, or weeping is a good idea. Polypropylene trays are suitable for most purposes. Store oxidizers, reducing agents, and fuels separately to prevent contact in the event of an accident.
6.8.1 Chemical Compatibility in Storage
Keep incompatibles separate during transport, storage, use, and disposal18. Chemicals in containers having a capacity of more than 2 kg or 2 liters shall be stored only with other compatible chemicals.19 Consult SDS for chemical compatibility information. Further information can be found in Bretherick’s Handbook of Reactive Chemical Hazards (Urben & Pitt, 2017), an extensive compendium that is the basis for lists of incompatible chemicals included in other reference works.
It is most important to separate compatible pyrophoric and water-reactive chemicals and chemicals that are incompatible with all other storage groups. These two groups merit their own storage cabinets. Always store fuels away from oxidizers.
Concentrated oxidizing agents are incompatible with concentrated reducing agents. Nitric acid and organic compounds together present a dangerous fire risk. Carcinogenic chemicals can be stored with others of a similar grouping based on their properties.
Take care not to mix incompatible waste. This is a special concern with commingled waste solvents, which must be chemically compatible to ensure that heat generation, gas evolution, or another reaction does not occur.
Compatible Organic Bases
- Diethylamine
- Piperidine
- Triethanolamine
- Benzylamine
- Benzyltrimethylammonium hydroxide
Compatible Pyrophoric & Water-Reactive Materials
- Sodium borohydride
- Benzoyl chloride
- Zinc dust
- Alkyl lithium solutions such as methyl lithium in tetrahydrofuran
- Methanesulfonyl chloride
- Lithium aluminum hydride
Compatible Inorganic Bases
- Sodium hydroxide
- Ammonium hydroxide
- Lithium hydroxide
- Cesium hydroxide
Compatible Organic Acids
- Acetic acid
- Citric acid
- Maleic acid
- Propionic acid
- Benzoic acid
Compatible Oxidizers Including Peroxides
- Nitric acid
- Perchloric acid
- Sodium hypochlorite
- Hydrogen peroxide
- 3-Chloroperoxybenzoic acid
Compatible Inorganic Acids not Including Oxidizers or Combustibles
- Hydrochloric acid
- Sulfuric acid
- Phosphoric acid
- Hydrogen fluoride solution
Poisonous Compressed Gases
- Sulfur dioxide
- Hexafluoropropylene
Compatible Explosives or Other Highly Unstable Materials
- Picric acid dry(<10% H2O)
- Nitroguanidine
- Tetrazole
- Urea nitrate
Nonreactive Flammables and Combustibles, Including Solvents
- Benzene
- Methanol
- Toluene
- Tetrahydrofuran
Incompatible with ALL Other Storage Groups
- Picric acid moist (10-40% H2O)
- Phosphorus
- Benzyl azide
- Sodium hydrogen sulfide
6.8.2 Inspection of Stored Chemicals
Storage Area Inspections
Chemical storage areas shall be inventoried and inspected at least annually and any unwanted or expired chemicals shall be removed. During this inspection, the list of chemicals present in the laboratory shall be updated or verified and the date and name of the inspector recorded.
Container Inspections
Although the deterioration in storage of a specific compound cannot be predicted in detail, generalizations can often be made about the reaction characteristics of groups of compounds. Some general conclusions about the stability of classes of chemicals can be reached, and corresponding storage time spans can be identified. Visual inspection of stored chemicals is important in the disposal decision.
Chemicals showing any of the indications listed below shall be turned over to Research Safety for safe disposal:
- Slightly cloudy liquids
- Darkening or change in color
- Spotting on solids
- Caking of anhydrous materials
- Existence of solids in liquids or liquids in solids
- Pressure buildup in containers
- Evidence of reaction with water
- Corrosion or damage to the container
- Missing or damaged (i.e., illegible) labels
6.8.3 Refrigerator Storage
Flammable liquids shall not be stored in ordinary domestic refrigerators. Refrigerator temperatures are almost universally higher than the flash points of flammable liquids, and ignition sources are readily available inside the storage compartment. Furthermore, the compressor and its circuits are typically located at the bottom of the units, where vapors (from flammable liquid spills or leaks, for example) may easily accumulate.
Laboratories requiring refrigerator storage for flammable liquids shall purchase explosion-safe models that require no modification. Under no circumstances should lab members attempt to perform modification themselves.
Please note that “explosion-safe” refrigerators are not “explosion-proof.” “Explosion-proof” refers to refrigeration equipment that has been designed to protect against ignition of flammable vapors both inside and outside the storage compartment.
If refrigerators are not “explosion-safe” or “explosion-proof,” they shall be labeled “Caution. Not approved for flammable liquid storage.” Flammable liquids shall not be stored in cold rooms. Such storage facilities pose explosion hazards because the various control switches and defroster heaters can spark and ignite flammable vapors.
Chemicals stored in refrigerators or cold rooms shall be sealed and labeled with the name of the person who stored the material, in addition to the labeling requirements. Old chemicals shall be disposed of after a specified storage period.
Food shall not be stored in a refrigerator used for chemical storage. The refrigerator shall be labeled “Food Must Not Be Stored in This Refrigerator” or equivalent. Refrigerators used for food shall be marked “Food Only” or equivalent and shall not be in the work area.
6.9 Safety for Specific Chemical Operations
Operations that may generate airborne contaminants or that use flammable liquids or toxic, reactive, or odoriferous materials shall be conducted in a chemical fume hood or other appropriate containment enclosure. Whenever hazardous gases or fumes are likely to evolve, an appropriate trap, condenser, or scrubber shall be used to minimize release of material to the environment.
6.9.1 Assembling Apparatus
Apparatus should be set up well back from the edge of the work area, be it a bench or a hood. When assembled in a hood, apparatus should not obstruct the area. To avoid overflow, choose apparatus with at least 20 percent more capacity than would normally accommodate the volume of chemical planned for the operation. All parts of the apparatus shall be firmly balanced and supported. Tubing shall be fastened with wire or appropriate clamps.
Stirrer motors and vessels shall be positioned and secured to ensure proper alignment. Magnetic stirring is preferable, and non-sparking motors or air motors shall be used in any laboratory that might contain flammable vapors.
Funnels and other apparatus with stopcocks shall be firmly supported and oriented so that gravity will not loosen the stopcock plug. Use a retainer on the stopcock plug and lubricate glass stopcocks. Do not lubricate Teflon stopcocks.
Include a vent in apparatus for chemicals that are to be heated and place boiling stones in unstirred vessels. A pan under a reaction vessel or container will confine spilled liquids in the event of glass breakage.
The safety factor (bursting pressure/maximum operating pressure) for water hoses with a maximum working pressure of 10 bar shall be 3:1. The safety factor for gas hoses and tubing shall be 4:1. Wherever unsafe pressures can build up within an apparatus section or system leg, an over-pressure relief has to be built in.
6.9.2 Unattended/Overnight Operations
If experiments run while a researcher is not present, an Overnight Experiment Notice containing information about the experiment and the name of a contact person for emergencies shall be posted on the laboratory door. Forms are available from Research Safety.
Reactions that are left unattended for long periods of time or overnight are prime sources of fires, floods, and explosions. When equipment such as power stirrers, hot plates, heating mantles, and water condensers are run unattended or overnight only fail-safe designs must be used. Hotplates and ovens must be equipped with safe temperature limits set within 25C of the maximum experiment temperature. Other examples are flow monitors that will shut down equipment in case of water supply failure or fail-safe hose connectors.
At night, emergency personnel are entirely dependent on accurate instructions and information available at the laboratory space. Unplug heating mantles, hotplates and other heating devices that are not in use to avoid accidental heating of combustibles and flammables.
6.9.3 Extractions
Extractions can present a hazard because of the potential buildup of pressure from a volatile solvent and an immiscible aqueous phase. Glass separator funnels used in laboratory operations are particularly susceptible to problems because their stoppers or stopcocks can be forced out, resulting in a spill of the contained liquid. It is even possible for pressure to burst the vessel.
To use a separator funnel correctly, do not attempt to extract a solution until it is cooler than the boiling point of the extractant. When a volatile solvent is used, the un-stoppered separator funnel should first be swirled to allow some solvent to vaporize and expel some air. Close the funnel and invert it with the stopper held in place and immediately open the stopcock to release more air plus vapor. Do this with the hand extended around the barrel to keep the stopcock plug securely seated.
Point the barrel of the separator funnel away from yourself and others and vent it to the hood. Do not vent the funnel near a flame or other ignition source. Close the stopcock, shake with a swirl, and immediately open the stopcock with the funnel in the inverted position to vent the vapors again. If it is necessary to use a separator funnel larger than one liter for an extraction with a volatile solvent, the force on the stopper may be too great, causing the stopper to be expelled. Consider performing the extraction in several smaller batches.
6.9.4 Distillations20
Distillation processes can sometimes be avoided by purchasing smaller quantities and high-purity solvents.
Perform distillations in a chemical hood. Distillation of flammable and combustible solvents is dangerous due to the presence of heat, flammable vapors and the build up of peroxides to explosive levels. Distillations should be maintained under inert atmosphere. At the completion of vacuum distillations, backfill the apparatus with inert gas. Stills in use should be attended at all times and should have an automatic high-temperature shutoff.
Certain common laboratory chemicals form peroxides on exposure to oxygen in air. Over time, some chemicals continue to build peroxides to potentially dangerous levels, whereas others accumulate a relatively low equilibrium concentration of peroxide, which becomes dangerous only after being concentrated by evaporation or distillation. Because distillation of a stabilized solvent removes the stabilizer, the distillate must be stored with care and monitored for peroxide formation.
The chemicals below are a peroxide hazard on concentration (distillation/evaporation). A test for peroxide should be performed if concentration is intended or suspected.
| Acetal | Dioxane (p-dioxane) |
| Cumene | Ethylene glycol dimethyl ether (glyme) |
| Cyclohexene | Furan |
| Cyclooctene | Methyl acetylene |
| Cyclopentene | Methyl cyclopentane |
| Diaacetylene | Methyl-isobutyl ketone |
| Dicyclopentadiene | Tetrahydrofuran |
| Diethylene glycol dimethyl ether (diglyme) | Tetrahydronaphthalene |
| Diethyl ether | Vinyl ethers |
Column Purification Systems or “Push Stills”
Column purification systems offer a safer, more environmentally friendly process for providing dry, oxygen-free, high-purity solvents as compared with thermal distillation. The level of impurity (water, oxygen, peroxides) is comparable to thermal distillation. The system is usually composed of refillable stainless steel “kegs” that hold high-purity solvent and act as a solvent reservoir. Inert gas (nitrogen, argon) is used to maintain an inert atmosphere as well as to force solvent through the packed columns that contain activated alumina (for water scavenging) and copper catalyst (for oxygen scavenging). For those solvents that are incompatible with copper (e.g., tetrahydrofuran, methylene chloride, acetonitrile), a second column of alumna is used along with a dry nitrogen or argon purge to facilitate oxygen removal. The solvent product is dispensed from the columns into a variety of specialized containers for use in the laboratory (glass, stainless steel, etc.).
Column purification systems present much less of a fire risk compared with thermal distillation, because they do not employ heating devices or reactive metals. Because glass containers are not needed, the potential for injury or spill related to breakage is also eliminated.
There is no need for heating mantles when solvent is present, and the intrinsically safe properties of the system allow it to be set up virtually anywhere in the laboratory, thus eliminating the need to place the apparatus in a chemical hood. As a result, there is a significant savings in electricity usage, although heating jackets may be required for installations where the water and oxygen scavengers are activated or regenerated. When using a column purification system, it is important not to draw down the column completely empty. Bubbling or splattering as the product is drawn from the column is an indication of breakthrough of argon. For the column to be functional again, a lengthy priming operation may be needed.
Peroxide-forming solvents such as tetrahydrofuran and diethyl ether that are processed and dispensed via a purification system shall be regularly tested for peroxide concentrations. A purification system that will not be used in the foreseeable future or that will be placed into storage should be emptied of its solvent contents.
Solvent Stills
Most high-purity solvents are commercially available in specialized kegs or may be obtained from column purification systems; thus, thermal distillation processes should be a last resort. There have been numerous fires attributed to solvent stills, some resulting in serious injuries and extensive damage to the laboratory spaces. [See, e.g., Yarnell (2002).]
Solvent stills are used to produce dry, high-purity solvents. The process involves reflux and distillation of organic solvents (many of which are flammable liquids) over drying materials, under nitrogen or argon gas. The most commonly used drying agents involve potentially pyrophoric metals: sodium metal/benzophenone and magnesium metal/iodine. The stills must be periodically quenched to prepare the still bottoms for disposal. This usually involves adding solvent to consume the scavenging agents. The process itself poses a risk of reactive metal adhering to the bottom of the flask, with the potential for exposure to air causing a spontaneous fire. Most thermal stills rely on electric heating mantles to heat the flammable solvents upward of 82 °C (180 °F), presenting a fire risk and potential ignition source.
Always set up stills in a chemical hood. Although many procedures suggest allowing the process to run overnight, it is prudent to ensure that it is not left completely unattended. Start the process at the beginning of the day and let it run as long as laboratory members are present. Place shields around the still to protect members in the event of a serious accident. Deactivate the stills under argon or nitrogen, never air. Do not add fresh solvent, drying agent, or indicator while the still is hot. Ensure that water cooling lines are in good condition. Do not allow material to accumulate at the bottom of the still; quench the still at the end of every procedure and clean thoroughly. Use caution when collecting the reactive materials as waste.
6.9.5 Temperature Control
Since the rates of most reactions accelerate as the temperature increases, highly exothermic reactions can become violent without adequate cooling. Viscous liquids transfer heat poorly and require special precautions. Apparatus shall be assembled so that either heating or cooling can be applied or withdrawn readily.
21Whenever an electrical heating device is used, either a temperature controller or a temperaturesensing device must be used that will turn off the electric power if the temperature of the heating device exceeds some preset limit. Similar control devices are available that will turn off the electric power if the flow of cooling water through a condenser is stopped owing to the loss of water pressure or loosening of the water supply hose to a condenser. Independent temperature sensors must be used for the temperature controller and shutoff devices. Fail-safe devices, which can be either purchased or fabricated, can prevent the more serious problems of fires or explosions that may arise if the temperature of a reaction increases significantly because of a change in line voltage, the accidental loss of reaction solvent, or loss of cooling. Use fail-safe devices for stills purifying reaction solvents, because such stills are often left unattended for significant periods of time. Temperature-sensing devices absolutely must be securely clamped or firmly fixed in place, maintaining contact with the object or medium being heated at all times. If the temperature sensor for the controller is not properly located or has fallen out of place, the controller will continue to supply power until the sensor reaches the temperature setting, creating an extremely hazardous situation.
Insert a thermometer in heated liquids if dangerous exothermic decomposition is possible. This will provide a warning and may allow time to remove the heat and apply external cooling.
A more thorough hazard review should be done when reaction temperatures (> 150 °C; < -30 °C), the pressure within a reaction vessel can be expected to exceed 10 bar, or reagents are fed at >2 bar of pressure.22
6.9.6 Heat Blocks, Oil and Sand Baths
Heat blocks, specifically sized for round bottom flasks, can be placed directly on a stirring hotplate. Heat blocks are a good replacement for oil baths or heating mantles.

Improper use of a hot oil or sand bath may create serious hazards such as an overturned bath, spatter from water falling into the bath, smoke caused by decomposition of the oil or organic materials in the oil, and fire from overheating the oil. Baths shall not be left unattended without a high-temperature shutoff. The oil shall be properly labeled, including information on its safe working temperature. Fresh silicone oils have higher auto ignition temperatures and are recommended over the use of paraffin oil. Contact with oxygen and long exposure to temperature at the upper end of their application range accelerates the degradation of silicone oils and will lower the auto ignition temperature. Replace the silicone oil in openly heated oil baths at least annually.
23Contain heated oil in either a metal pan or a heavy-walled porcelain dish; a Pyrex dish or beaker can break and spill hot oil if struck accidentally with a hard object. Mount the oil bath carefully on a stable horizontal support such as a laboratory jack that can be raised or lowered easily without danger of the bath tipping over. Always clamp equipment high enough above a hot plate or oil bath that if the reaction begins to overheat, the heater can be lowered immediately and replaced with a cooling bath without having to readjust the clamps holding the equipment setup. Never support a bath on an iron ring because of the greater likelihood of accidentally tipping the bath over. Provide secondary containment in the event of a spill of hot oil. Wear proper protective gloves when handling a hot bath. Where only stirring of combustible or flammable liquids is use a stirrer instead of a stirring hotplate to avoid accidental heating.
6.9.7 Cooling Baths24
The preferred liquids for dry-ice cooling baths are isopropyl alcohol or glycols; add dry ice slowly to the liquid portion of the cooling bath to avoid foaming. Avoid the common practice of using acetone– dry ice as a coolant; the alternatives are less flammable, less prone to foaming and splattering with dry ice, and less likely to damage some trap components (O-rings, plastic). Dry ice and liquefied gases used in refrigerant baths should always be open to the atmosphere. Never use them in closed systems, where they may develop uncontrolled and dangerously high pressures.
Exercise extreme caution in using liquid nitrogen as a coolant for a cold trap.
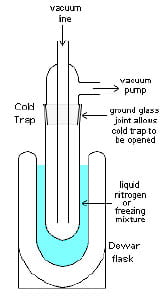
If such a system is opened while the cooling bath is still in contact with the trap, oxygen may condense from the atmosphere. The oxygen could then combine with any organic material in the trap to create a highly explosive mixture. Therefore, do not open a system that is connected to a liquid nitrogen trap to the atmosphere until the liquid nitrogen Dewar flask or container has been removed. A liquid nitrogen– cooled trap must never be left under static vacuum. Also, if the system is closed after even a brief exposure to the atmosphere, some oxygen may have already condensed. Then, when the liquid nitrogen bath is removed or when it evaporates, the condensed gases will vaporize, producing a pressure buildup and the potential for explosion. The same explosion hazard can be created if liquid nitrogen is used to cool a flammable mixture that is exposed to air. Caution must be applied when using argon, for instance as an inert gas for Schlenk or vacuum lines, because it condenses as a colorless solid at liquid nitrogen temperature. A trap containing frozen argon is indistinguishable from one containing condensed solvent or other volatiles and presents an explosion hazard if allowed to warm without venting.
6.9.8 Laboratory Reactor Pressure Vessels
Laboratory reactor pressure vessels may also be referred to as sample preparation bombs, acid digestion bombs, hydrothermal reactors or chemical digestion autoclaves.25
 |
 |
Heating chemicals inside a closed vessel can result in some of the highest gas or super critical fluid pressures encountered in a laboratory space.
Novice users must be directly supervised in experimental design, vessel assembly and heating mode selection until they exhibit full understanding and proficiency.
As part of a laboratory reactor pressure vessel safety program, write detailed standard operating procedures, including intended operating pressures and temperatures, and upload to the Lumen Profile. Contact Research Safety for experiments that are intended to build up pressure above 500psia (34 bar).26
If you use laboratory reactor pressure vessels in your experiments, it’s important to understand what conditions increase the hazards associated with use so you can prevent dangerous ruptures or explosions from occurring. All laboratory reactor pressure vessels shall be equipped with a form of overpressure relief to protect the vessel from the hazards of unexpected or dangerously high internal pressures. Appropriate over pressure relief through a safety rupture disk or safety relief valve must be part of the laboratory reactor pressure vessel design.
- DO NOT use a laboratory reactor pressure vessel without overpressure relief.
- DO NOT use laboratory reactor pressure vessels without manufacturer’s documentation of maximum pressure and temperature.
- DO NOT assemble or maintain pressure vessels without manufacturer’s literature.
- DO NOT exceed temperature limits for reactions or pressure vessels specifications.
- DO NOT exceed vessel loading limits.
- DO NOT form explosive materials inside a pressure vessel.
Some chemicals and mixtures are prohibited in laboratory reactor pressure vessels.
- DO NOT treat fats, fatty acids, glycerin and similar materials with nitric acid in pressure vessels.
- DO NOT treat cellulosic materials with mixed nitric and sulfuric acids.
- DO NOT use perchloric acid, picric acid or concentrated hydrazine in these vessels
- AVOID reactions which are highly exothermic or which may be expected to release large volumes of gas.
Overloading of a pressure vessel is a significant hazard. Where available, identify the charging limits for each chemical and vessel size in the manufacturer’s literature. Always evaluate the stoichiometry and chemistry that you are trying to achieve with special considerations for catalysts and gaseous by-products that may affect pressure build up inside the vessel. Assess any intermediates, side-products and products that may form and their behavior within the vessel, including their corrosive nature and their tendency to violently decompose at elevated temperature and pressure. Determine maximum temperature and pressure limits expected, taking into account the energetics of the reaction being conducted and any pathways that might cause the reaction to run out of control. A formal written risk assessment is strongly encouraged.
Defective temperature controls or operator inattention can be the cause of dangerous overheating. In order to prevent dangerous overheating, the best practice is to:
- Use ovens or heating devices with high temperature limit controllers.
- Heat general purpose metal body laboratory reactor pressure vessels only in an oven.
- Heat polymer body laboratory reactor pressure vessels only in a microwave oven.
- Heat other laboratory reactor pressure apparatus behind a blast shield or suitable barrier.
- Post caution signs or an Overnight Experiment Notice when heating pressure vessels unattended.
Some pressure vessels are equipped with a polytetrafluoroethylene (PTFE) cup and lid liner. Due to PTFE flow, once a PTFE cup and lid is pressurized it becomes a uniquely matching pair. Using unmatched pairs of cups and lids will cause leaks. Store all the parts of a pressure vessel together to avoid mismatches. Periodically conduct a leak check in accordance with the manufacturer’s literature.
Not all pressure vessels use a PTFE insert. Internal wetted parts of a pressure vessel have to be constructed resistant to corrosive materials at the expected operating pressure. Each alloy has its own physical strength and temperature characteristics as well as its own unique resistance to certain corrosive materials. All of these factors must be considered when making a selection. Dedicate pressure vessels for either acid or base service. Do not interchange the use of acids and bases in the same pressure vessel.
Register research activity involving the use of laboratory reactor pressure vessels in the Lumen Profile. Principal investigators must assure user competency. Use Lumen to assign lab member training for basic knowledge competency. For further information and guidance contact Research Safety.
Research Safety reserves the right to take possession of any pressure vessel deemed unsafe due to noncompliance with the safety guidelines highlighted above.
6.9.9 Reduced Pressure Operations
Protect vacuum desiccators by covering them with cloth-backed friction or duct tape, elastic mesh netting or shielding them for protection in case of implosion. Vacuum lines shall be trapped and shielding used whenever apparatus is under reduced pressure. Only chemicals being dehydrated should be stored in a desiccator. Before opening a desiccator that is under reduced pressure, make sure that atmospheric pressure has been restored.
Water aspirators for reduced pressure are used mainly for filtration purposes, and only equipment that is approved for this purpose should be used. Never apply reduced pressure to a flat-bottomed flask unless it is a heavy-walled filter flask designed for that purpose. Place a trap and a check valve between the aspirator and the apparatus so that water cannot be sucked back into the system if the water pressure falls unexpectedly during filtering. This also applies to rotary evaporation equipment that use water aspirators for reduced pressure.
If vacuum pumps are used, place a cold trap between the apparatus and the vacuum pump so that volatiles from a reaction or distillation do not get into the pump oil or out into the atmosphere. Exhausts from pumps shall be vented to a hood or ventilation system. Pumps with belt drives must be equipped with belt guards to prevent hands, hair, or loose clothing from being caught in the belt pulley.
Desiccators27
If a glass vacuum desiccator is used, it should be made of Pyrex or similar glass, completely enclosed in a shield, encased in mesh netting, or wrapped with friction tape in a grid pattern that leaves the contents visible and at the same time guards against flying glass if the vessel implodes. Plastic (e.g., polycarbonate) desiccators reduce the risk of implosion and may be preferable but should also be shielded while evacuated. Solid desiccants are preferred. Never carry or move an evacuated desiccator. Take care opening the valve to avoid spraying the desiccator contents from the sudden inrush of gas.
Rotary Evaporators28
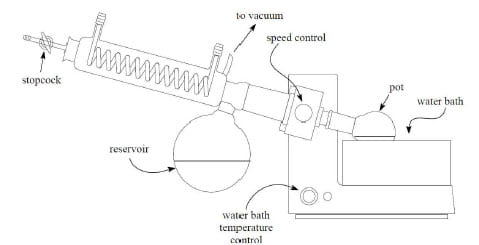
29
Glass components of the rotary evaporator should be made of Pyrex or similar glass. Glass components such as the reservoir and condenser/cooling finger should either be coated with a layer of safety plastic resin or protected with plastic mesh netting to contain glass debris in case of implosion. Completely enclose in a shield to guard against flying glass should the components implode. Gradually increase rotation speed and application of vacuum to the flask whose solvent is to be evaporated.
6.9.10 Cold Traps30
Cryogenic liquids are materials with boiling points of less than −73 °C (−100 °F). Liquid nitrogen, argon, and slush mixtures of dry ice with isopropyl alcohol are the materials most commonly used in cold traps to condense volatile vapors from a gas or vapor stream. Cold traps used in reduced pressure systems should be placed in stainless steel cryogen dewar flasks . If this option is not possible, the cold traps should be coated with plastic resin or wrapped with cloth-backed friction or duct tape. In the event of an implosion, the coating will reduce the amount of flying glass.
Users of cold traps should be aware of the boiling points of the components and the possible materials that can condense in the reduced-pressure system. After completion of an operation in which a cold trap has been used, isolate the trap from the source, remove from the coolant, and vent to atmospheric pressure in a safe and environmentally acceptable way. Otherwise, pressure could build up, creating a possible explosion or sucking pump oil into a vacuum system. Dry ice and liquefied gases used in refrigerant baths should always be open to the atmosphere. Never use them in closed systems, where they may develop uncontrolled and dangerously high pressures.
Exercise extreme caution in using liquid nitrogen as a coolant for a cold trap. If such a system is opened while the cooling bath is still in contact with the trap, oxygen may condense from the atmosphere. Caution must be applied when using argon, for instance as an inert gas for Schlenk or vacuum lines, because it condenses as a colorless solid at liquid nitrogen temperature. A trap containing frozen argon is indistinguishable from one containing condensed solvent or other volatiles and presents an explosion hazard if allowed to warm without venting.
6.9.11 Transporting Chemicals In-House
The precautions that should be followed to protect colleagues, non-laboratory personnel, and facilities when you transport chemicals in University buildings are listed below.
- Use secondary containers. The container-within-a-container concept will protect the primary containers from shock during any sudden change of movement. Secondary containment is especially important when chemicals are moved in public areas, such as hallways or elevators, where the effects of a spill would be more severe. Transport large containers of corrosives in a chemically-resistant bucket or other container designed for this purpose.
- Always use a sturdy cart, and make sure the cart has a low center of gravity. Carts with large wheels are best for negotiating irregularities in floors and at elevator doors.
- Use freight elevators for moving chemicals and biological materials. Passenger elevators shall not be used for this purpose. Elevator transports of hazardous waste or cryogenic liquids shall only be accompanied by the person transporting the materials – no public.
- Do not transport incompatible chemicals in container sizes >2kg or 2 liters together on the same cart31.
- Only transport gas cylinders with the valve-protection cap on. Only transport toxic gases with the valve-protection cap and the valve outlet cap on.
- All chemical containers being transported shall have labels identifying the contents.
6.10 Chemical Inventory
Chemical owners who use or store hazardous chemicals at Northwestern University are required by OSHA and fire regulation to maintain a chemical inventory for compliance with various safety and environmental regulations, and to provide critical information to responders during an emergency.
Northwestern laboratory personnel are responsible for maintaining an accurate chemical inventory for their laboratory. Each research laboratory has a corresponding ChemTracker inventory linked to its Lumen Profile. All laboratories that store chemicals are strongly encouraged to use ChemTracker to manage their inventory. Laboratories may use other chemical inventory programs or systems, however electronic copies of these inventories should be uploaded to their Lumen Profile.
Order only the amounts of stock necessary to support the research. Excess should not be purchased because it increases hazardous waste volume and raises the risk of significant spills.
Minimal stock also protects the safety of emergency responders. The greater the volume of chemicals on hand, the greater the risk that breakage of containers could lead to mixing of incompatible chemicals or release of reactive compounds.
6.10.1 Quantity Limits for Corrosives
The regulations for the Evanston Campus differ from the regulations on the Chicago Campus. The Northwestern University buildings on the Evanston Campus generally have to comply with the limits established in the International Building Code (IBC) and the International Fire Code (IFC). Research laboratories generally are in buildings classified as business occupancy (B). Groups of research laboratories within a building may be subdivided into fire control areas. For more specific information regarding laboratory design and building fire control specifications contact Facilities.
The chemical control zones maximum allowable quantities (MAQ) for corrosives are several hundred liters. Laboratory scale work is not expected to exceed these quantities. One exception is the MAQ for highly concentrated nitric acid. Consult Research Safety before acquisition of more than 4 liters of nitric acid in excess of 68%.
Chicago Campus
Contact Research Safety for further information on the storage of corrosives if you intend to store more than 40 liters or a single container size exceeding 4 liters.
6.10.2 Quantity Limits for Highly Reactives and Toxics
Table 6.10.2 Evanston Campus
International Fire Code (IFC) 2018 – Maximum Allowable Quantities (MAQ) in Storage per Sprinklered Fire Control Areas Unless Constructed to High Hazard Group (H-2 or H-3) Specifications (in pounds)
| HAZARDOUS MATERIAL | NFPA CLASS | GROUND FLOOR-3RD FLOOR (100% MAQ) | BASEMENT AND 4TH-6TH LEVEL ABOVE GRADE (75% MAQ) | SUBBASEMENT AND 7TH LEVEL ABOVE GRADE (50% MAQ) |
|---|---|---|---|---|
| Highly Toxic | 20 | 15 | 10 | |
| Toxic | 1,000b | 750b | 500b | |
| Organic Peroxide | UD (Highest Hazard Class) | 2b | 1.5b | 1b |
| Organic Peroxide | I | 10b | 7.5b | 5b |
| Organic Peroxide | II | 100b | 75b | 50b |
| Organic Peroxide | III | 250b | 185b | 125b |
| Oxidizer | 4 | 2b | 1.5b | 1.0b |
| Oxidizer | 3 | 20b | 15b | 10b |
| Oxidizer | 2 | 500b | 375b | 250b |
| Unstable reactive | 4 | 2b | 1.5b | 1b |
| Unstable reactive | 3 | 10b | 7.5b | 5b |
| Unstable reactive | 2 | 100b | 75b | 50b |
| Water reactive | 3 | 10b | 7.5b | 5b |
| Water reactive | 2 | 100b | 75b | 50b |
| Explosives | Consult with Research Safety before ordering | |||
|
b. Quantities shall be increased 100% when stored in approved cabinets, gas cabinets, exhausted enclosures, or safety cans as specified by the International Fire Code. |
||||

Chicago Campus
All research buildings on the Northwestern University Chicago Campus are equipped with automatic sprinkler systems. The McGaw/Olson building is classified as institutional occupancy NOT business occupancy, which limits the storage quantities in accordance with NFPA 99. Contact Research Safety for further information on the storage of highly reactive chemicals.
6.10.3 Quantity Limits for Flammables
Evanston Campus
The regulations for the Evanston Campus differ from the regulations on the Chicago Campus. Generally, the quantity limits are higher in buildings equipped with automatic fire sprinklers. The research buildings on the Northwestern University Evanston Campus equipped with automatic sprinkler systems are:
TABLE 6.10.3a
| BUILDING | ADDRESS |
|---|---|
| Pancoe-NUH Life Sciences Pavilion | 2200 Campus Drive |
| Cook Hall | 2220 Campus Drive |
| Ryan Hall | 2190 Campus Drive |
| Technological Institute | 2145 Sheridan Rd |
| Ford Motor Company Engineering Design | 2133 Sheridan Rd |
| Frances Searle Building | 2240 Campus Drive |
| Mudd Hall | 2233 Tech Drive |
| Silverman Hall | 2170 Campus Drive |
| 1801 Maple Avenue | 1801 Maple Avenue |
Note, that the Hogan building is not fully equipped with automatic sprinkler systems.
The Northwestern University building occupants on the Evanston Campus generally have to comply with the limits established in the International Building Code (IBC) and the International Fire Code (IFC). Research labs generally are in buildings classified as business occupancy (B). Groups of research laboratories within a building may be subdivided into fire control areas. For more specific information regarding laboratory design and building fire control specifications contact Facilities.
The total quantities of flammable or combustible liquids allowed in a fire control area are limited by the floor level above grade and other construction and use specifications. Typical research laboratories are not constructed to high hazard group specifications.
The following table shows the maximum allowable quantities that can be stored in a single fire control area per floor.
TABLE 6.10.3b
International Fire Code (IFC) 2018 – Maximum Allowable Quantities in Storage per Sprinklered Fire Control Area Unless Constructed to High Hazard Group (H-2 or H-3) Specifications (in Gallons)
| HAZARDOUS MATERIAL | NFPA CLASS | GROUND FLOOR TO 3RD FLOOR (100% MAQ) | BASEMENT AND 6TH FLOOR ABOVE GRADE (75% MAQ) | SUBBASEMENT AND 7TH FLOOR ABOVE GRADE (50% MAQ) |
|---|---|---|---|---|
| Flammable Liquid | IA IB IC |
60b 120b 180b |
40b 90b 135b |
30b 60b 90b |
| Combination of Flammable Liquids | IA+IB+IC | 240b,c,d | 180b,c,d | 120b,c,d |
| Combustible Liquid | II IIIA IIIB |
240b,d 660b,d 26,400b,d |
180b,d 495b,d 19,800b,d |
120b,d 330b,d 13,200b,d |
| Pyrophoric | Not applicable | 48 poundsb,e | 6 poundsb,e | 4 poundsb,e |
|
b. Quantities shall be increased 100% when stored in approved cabinets, gas cabinets, exhausted enclosures, or safety cans as specified by the International Fire Code. Where note d applies, the increase for both shall be applied accumulatively. c. Except for IFC “Spill control for hazardous material liquids. Rooms, buildings or areas used for the storage of hazardous material liquids in individual vessels having a capacity of more than 55 gallons, or in which the aggregate capacity of multiple vessels exceeds 1,000 gallons shall be provided with spill control to prevent the flow of liquids into adjoining areas.” d. Allowed only in buildings equipped throughout with an approved automatic sprinkler system in accordance with IFC 2003 Section 903.3.1.1. Note, that the Hogan and Catalysis buildings are not equipped with automatic sprinkler systems. e. Allowed only in buildings equipped throughout with an approved automatic sprinkler system in accordance with IFC. |
||||
Quantity limits on the Chicago Campus32
The regulations for the Chicago campus differ from the regulations on the Evanston campus. All research buildings on the Chicago campus are equipped with automatic sprinkler systems. With the exception of flammable liquids storage rooms by the loading docks of the Tarry and Lurie buildings, there are currently no rooms designed to the specifications of a dedicated flammable liquids storage room.
In the Chicago campus research spaces, a single container capacity for NFPA Class I flammable liquids is limited to 4 liters. The Laboratory Unit Fire Hazard Class is limited to Class C – low fire hazard. This means that the maximum quantity of Class I flammable liquids, in fire rated storage cabinets, is limited to 15 liters per 9.3 m2 (100 sf) of research space. Consult with Research Safety if Class II combustible liquids exceed 240 liters or Class III combustible liquids exceed 480 liters.
Whenever the amount of flammable liquids stored in a building exceeds the maximum aggregate amount, the excess flammable liquid shall be stored in a special room for flammable liquids.
Note, the McGaw/Olson building is classified as institutional occupancy NOT business occupancy, which limits the storage quantities in accordance with NFPA 99 Flammable and Combustible Liquids 11.7.2.3.1
In the McGaw/Olson laboratories, a maximum of 4 liters of combinations of Class I, II, and IIIA flammable liquids per 100 sq.ft. of laboratory space are allowed outside of a flammable liquids cabinet. Combinations of Class I, II, and IIIA may not exceed 8 liters per 100 sq ft in storage.
7.0 Hazards of Chemical Groups
7.1 Corrosives: Acids and Bases
![]()
Under hazardous waste regulation a pH <2 or >12.5 indicates corrosive characteristics. Corrosive acids and bases attack the skin and can cause permanent damage to the eyes. Therefore, exercise great care in attempting neutralization.
All the hydrogen halide acids are serious respiratory irritants. Hydrofluoric (HF) acid poses a special danger; both its gas and solutions are toxic, and it is rapidly absorbed through the skin, penetrating deeply into the body tissues. Contact with dilute solutions of hydrofluoric acid may cause no pain for several hours but result in serious burns. In all cases, immediate and thorough flushing with water for 5 minutes, followed by calcium gluconate antidote gel application and prompt attention by a physician are necessary.
Oxyacids such as sulfuric and nitric acid have widely differing properties. Sulfuric acid is a very strong dehydrating agent. When preparing solutions, always add the acid to water and remember that the heat of solution may produce a large increase in temperature. Nitric acid is a strong oxidizing agent that acts rapidly and turns exposed skin yellow to brown as a denaturing reaction occurs. Paper that has been used to wipe up nitric acid spills can ignite spontaneously when dry and should not be thrown into a wastebasket until first rinsed with water and neutralized.
Chromic acid is generally prepared as a cleaning solution; Research Safety recommends the use of replacement cleaners without chromium, which is carcinogenic. All chromic acid waste shall be collected and disposed of through Research Safety. For information regarding chromic acid substitutes, contact Research Safety.
Perchloric acid is a powerful oxidizing agent that may react explosively with organic compounds and other reducing agents. Small quantities of perchloric acid <72% at room temp or below can be used in a regular chemical hood. Any work with perchloric acid heated above ambient temperature requires Research Safety approval. If heated, it shall be used only in a perchloric-acid hood of noncombustible construction. Special hood wash-down features may be required. Perchloric acid should be handled with extreme care and kept from organic matter to prevent a serious explosion. Beakers of fuming perchloric acid >86% shall be handled with tongs rather than rubber gloves. Perchloric acid hoods shall be washed down after every perchloric acid digestion.
Perchloric acid containers shall be stored in glass outer containers and shall not be stored on wood shelving, as drips or leaks may render the wood shock-sensitive. Keep perchloric acid bottles on glass or ceramic trays that are large enough to hold all the acid if the bottle breaks. Storage of perchloric acid containers should not exceed one year. Digest organic matter with nitric acid before addition of perchloric acid. Never heat perchloric acid with sulfuric acid because dehydration may produce anhydrous perchloric acid, which is explosive.
Perchlorate esters have the same shattering effect as nitroglycerine. Transition metal perchlorates are capable of exploding. Perchlorates shall not be used without prior consultation with Research Safety.
Concentrated Bases
The most common bases found in laboratory spaces include the alkali metal hydroxides and aqueous solutions of ammonia. Sodium and potassium hydroxides are extremely destructive to both skin and eye tissues. When concentrated solutions are prepared, the heat of solution can raise the temperature to dangerous levels. Because ammonia solution vapors are such strong irritants, they should be used only in a chemical fume hood.
Table 7.1
Procedure for Inorganic Acid Neutralization (Does not apply to chromic acid)
| APPLICABLE ACIDS | Hydrochloric, sulfuric |
|---|---|
| EQUIPMENT |
|
| CAUTION |
|
|
1. Prepare a large amount of an ice-water-and-base solution of one of the following:
2. Slowly stir acid (which has been diluted to about 5%) into the base solution until the pH reaches about 5 to 10. |
|
| Note: The pH of hydrochloric and sulfuric solutions poured down the drain shall be between 5 and 10 to avoid violating local, state, or federal regulations. Submit all other neutralized acids for hazardous waste disposal | |
7.2 Flammable and Combustible Liquids
Definitions
According to most fire codes and regulations, including those for laboratory spaces, a flammable liquid is a liquid with a flash point below 100°F and a vapor pressure not exceeding 40 psi (absolute) at 100°F; it is called a Class I liquid. A liquid with a flash point at or above 100°F is classified as a combustible liquid and may be referred to as a Class II or Class III liquid. See also OSHA Flammable and Combustible Liquids.
The U.S. Department of Transportation (DOT) and the U.S. Environmental Protection Agency (EPA) use a different definition. These agencies define flammable liquids as those with a flash point of 140°F or lower and combustible liquids as those with a flash point greater than 140°F but less than 200°F. DOT and EPA definitions apply primarily to chemicals in transit and hazardous waste.
Flash point is the minimum temperature at which the liquid gives off vapors in sufficient concentration to form an ignitable mixture with air. The classes of liquids are further divided into subclasses, depending on the flash points and boiling points of the liquids (Table 7.2A). The classifications are important because regulations governing storage and use of a liquid are largely based on the liquid’s flash point.
Flammable liquids shall be handled only in areas with no ignition sources and shall not be heated with open flames. If flammable liquids in metal containers or equipment are transferred, the equipment and containers shall be bonded to avoid static-generated sparks.
A more thorough hazard review should be done when flammable reagents are used where the flash point is <10°C, the lower flammable limit is <10%, the auto ignition temperature is <200°C, or the minimum ignition energy is <0.5mJ.33
Storage
Flammable liquids shall not be stored in ordinary refrigerators or cold rooms. If it is necessary to refrigerate flammable materials, “explosion-proof,” “explosion-safe” or flammablestorage refrigerators shall be used. Combustible liquids are less of a fire hazard, although a rise in temperature increases their evaporation rate and the potential for ignition. If the quantity of flammable liquids in storage exceeds 40 liters (including liquid waste), flammable-liquid storage cabinets shall be used.
Allowable Quantities
The maximum allowable size of containers and portable tanks for flammable and combustible liquids is shown in Table 7.2B. Although the table indicates that the maximum allowable size of glass containers for Class IA and Class IB are 500 ml and one liter respectively, the liquids may be stored in glass containers of not more than 4 liter capacity if the required liquid purity (such as ACS analytical reagent grade or higher) would be affected by storage in metal containers or if the liquid would cause excessive corrosion of the metal container.
Bonding and Grounding
When a flammable liquid is poured into or withdrawn from a metal drum, the drum and the secondary container shall be electrically bonded to each other and to the ground to avoid the possible buildup of a static charge. Quantities no larger than 4 liters34 can be transferred to a glass container. If the liquid is transferred from a metal container to glass, the metal container should be grounded. Greater than 20 liter drums of flammable liquids are not permitted in laboratory spaces unless the quantity is necessary for daily use and is approved by Research Safety. In Evanston, transfer of a flammable liquid by gravity from a drum or carboy is permitted only through a self-closing valve or faucet. Chicago Fire Code for Flammable Liquids prohibits gravity transfer and requires that the liquid be transferred by pumping from an opening in the top of the container.
TABLE 7.2 A – Flammable Liquid Classification
| CLASS | FLASH POINT (°F) | BOILING POINT (°F) |
|---|---|---|
| IA | Below 73 | Below 100 |
| IB | Below 73 | At or above 100 |
| IC | At or above 73, below 100 | NA |
| II | At or above 100, below 140 | NA |
| IIIA | At or above 140, below 200 | NA |
| IIIB | At or above 200 | NA |
TABLE 7.2 B – Maximum Allowable Size of Flammable and Combustible Liquid Containers in Laboratory Spaces
| FLAMMABLE LIQUIDS | COMBUSTIBLE LIQUIDS | ||||
|---|---|---|---|---|---|
| CONTAINER | CLASS IA | CLASS IB | CLASS IC | CLASS II | CLASS IIIA |
| Glass | 500 mla | 1 litera | 4 liters | 4 liters | 20 liters |
| Metal (other than DOT drums) or Approved Plastic | 4 liters | 20 litersb | 20 litersb | 20 litersb | 20 liters |
| Safety Cans | 8 liters | 20 litersb | 20 litersb | 20 litersb | 20 liters |
| Metal Drum (DOT Spec.) | Not allowed | 20 litersb | 20 litersb | 240 litersb | 240 liters |
| Polyethylene | 4 liters | 20 litersb | 20 litersb | 240 litersb | 240 liters |
|
a. Glass containers of not more than 4 liters capacity are acceptable if the required purity would be adversely affected by storage in metal or if excessive corrosion would result from storage in metal. b. In instructional laboratory work areas, no container for Class I or II liquids shall exceed a capacity of 4 liters, other than safety cans which may be of 8 liters capacity. |
|||||
Reference: NFPA 45, Fire Protection for Laboratories Using Chemicals, National Fire Protection Association, 1996
7.3 Highly Reactive Chemicals
Highly reactive and explosive materials used in the laboratory require appropriate procedures.35
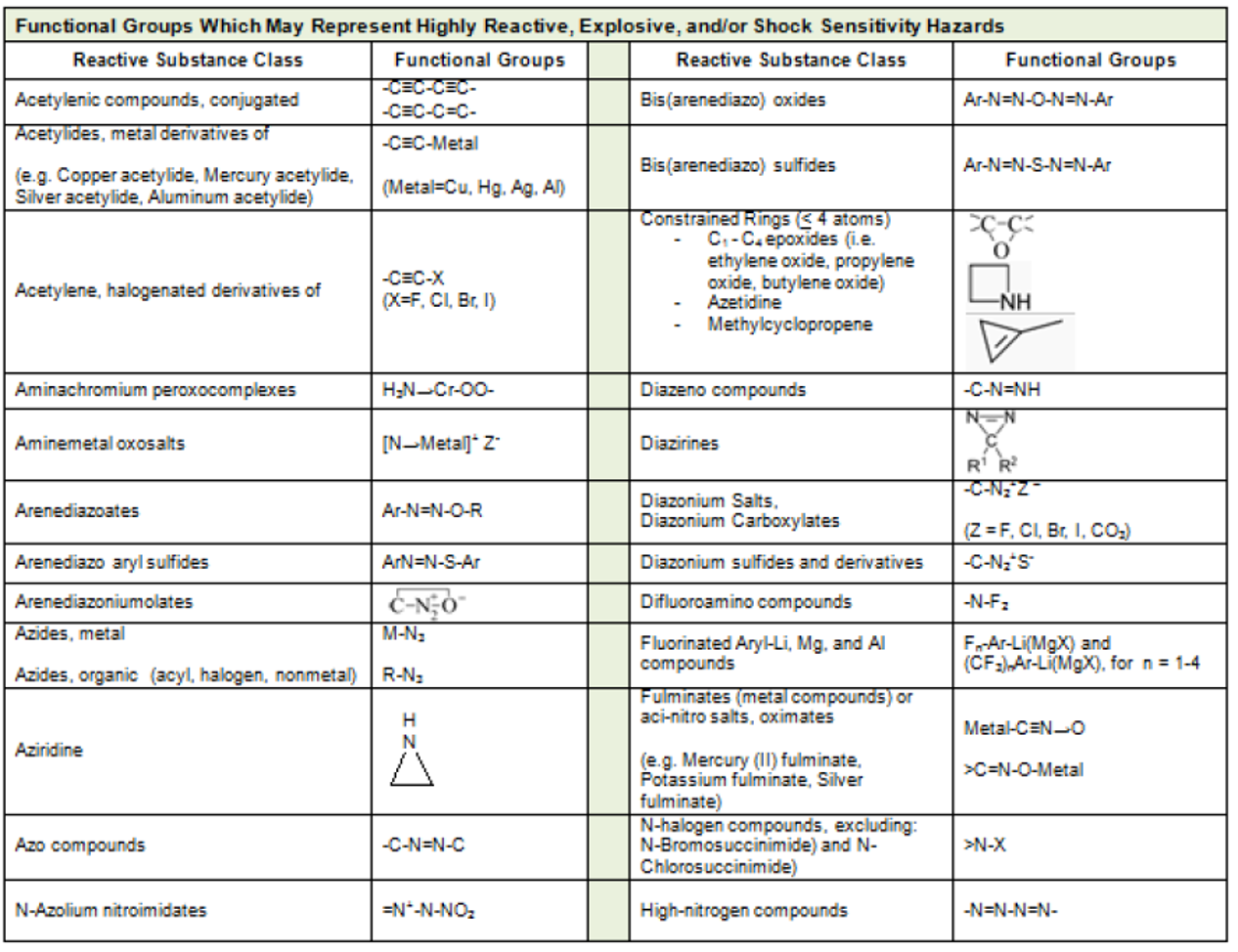
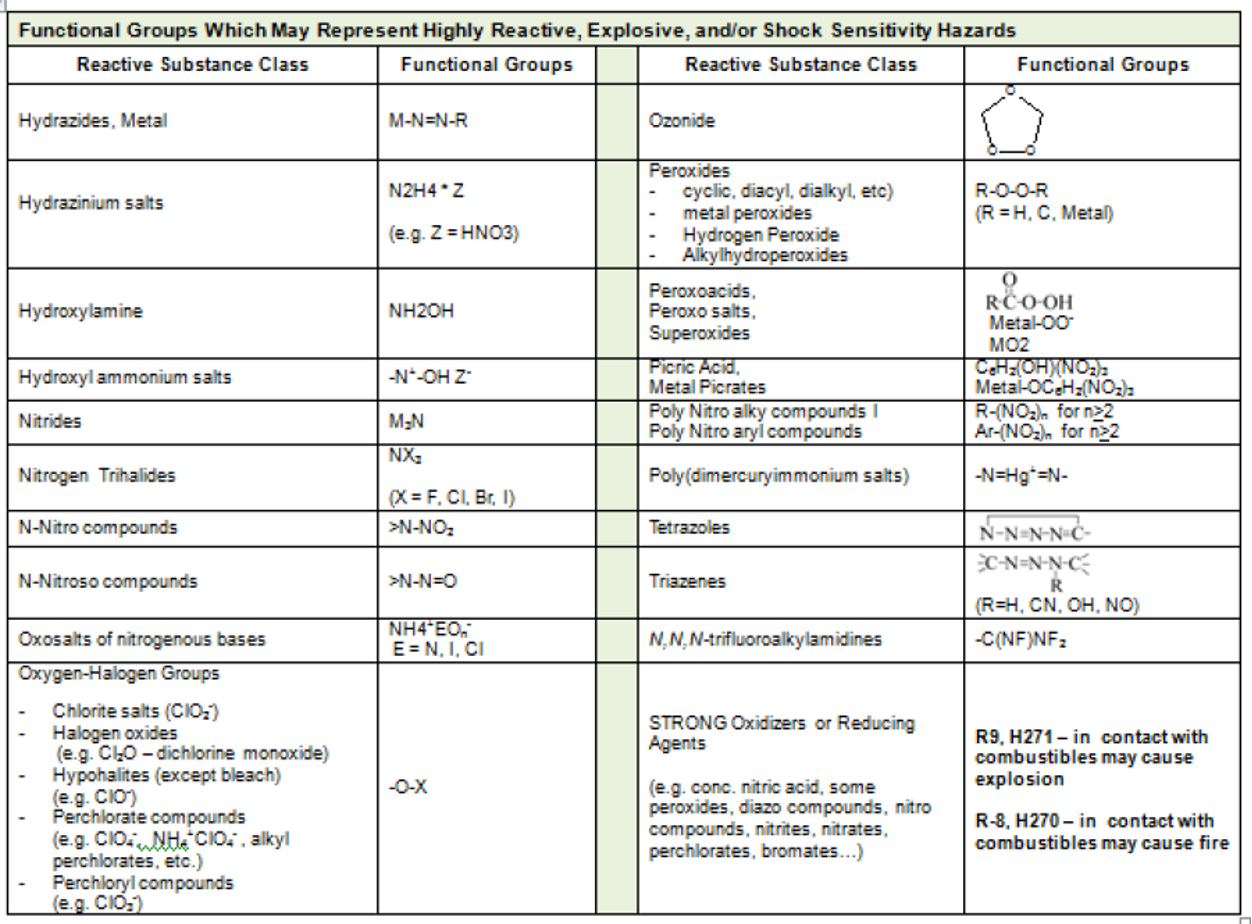
7.3.1 Organic Peroxides36
Organic peroxides are among the most hazardous chemicals normally handled in laboratory spaces. In addition to reading the SDS, consult the Bretherick’s Handbook of Reactive Chemical Hazards for more in depth information on reactive hazards and associated incidents. As a group, organic peroxides are flammable, low-power explosives and powerful oxidizers that are sensitive to shock, heat, sparks, friction, impact, and light. Many of them are much more shock-sensitive than typical explosives such as TNT.
Purchase and use of peroxides shall be kept to a minimum. Unused peroxides shall not be returned to the container. Glass containers with screw caps or glass stoppers shall not be used. Polyethylene bottles with screw caps are acceptable. Store liquid organic peroxides at the lowest possible temperature consistent with the solubility or freezing point. Liquid peroxides are particularly sensitive during phase changes. Follow the manufacturer’s guidelines.
Reduce the sensitivity of most peroxides to shock and heat by dilution with inert solvents, such as aliphatic hydrocarbons. However, do not use aromatics (such as toluene), which are known to induce the decomposition of diacyl peroxides.
Do not use solutions of peroxides in volatile solvents under conditions in which the solvent might vaporize because this will increase the peroxide concentration in the solution. See also Section 6.9.4 Distillations.
Do not use metal spatulas to handle peroxides because contamination by metals can lead to explosive decomposition. Magnetic stirring bars can unintentionally introduce iron, which can initiate an explosive reaction of peroxides. Ceramic, Teflon, or wooden spatulas and stirring blades may be used if it is known that the material is not shock sensitive.
7.3.2 Peroxide-Forming Chemicals37
Certain common laboratory chemicals form peroxides on exposure to oxygen in air. Essentially all compounds containing C—H bonds pose the risk of peroxide formation if contaminated with various radical initiators, photosensitizers, or catalysts. Over time, some chemicals continue to build peroxides to potentially dangerous levels, whereas others accumulate a relatively low equilibrium concentration of peroxide, which becomes dangerous only after being concentrated by evaporation or distillation. The peroxide becomes concentrated because it is less volatile than the parent chemical.
A related class of compounds includes inhibitor-free monomers prone to free radical polymerization that on exposure to air can form peroxides or other free radical sources capable of initiating violent polymerization. Note that care must be taken when storing and using these monomers—most of the inhibitors used to stabilize these compounds require the presence of oxygen to function properly, as described below. Always refer to the SDS and supplier instructions for proper use and storage of polymerizable monomers.
Excluding oxygen by storing potential peroxide formers under an inert atmosphere (N2 or argon) greatly increases the safe storage lifetime. Purchasing the chemical stored under nitrogen in septum capped bottles is also possible. In some cases, stabilizers or inhibitors (free-radical scavengers that terminate the chain reaction) are added to the liquid to extend its storage lifetime. Because distillation of the stabilized liquid removes the stabilizer, the distillate must be stored with care and monitored for peroxide formation. Furthermore, high-performance liquid chromatography–grade solvents generally contain no stabilizer, and the same considerations apply to their handling.
Types of Compounds Known to Autoxidize to Form Peroxides
- Ethers containing primary and secondary alkyl groups (never distill an ether before it has been shown to be free of peroxide)
- Compounds containing benzylic hydrogens
- Compounds containing allylic hydrogens (C=C—CH)
- Compounds containing a tertiary C—H group (e.g., decalin and 2,5-dimethylhexane
- Compounds containing conjugated, polyunsaturated alkenes and alkynes (e.g., 1,3- butadiene, vinyl acetylene)
- Compounds containing secondary or tertiary C—H groups adjacent to an amide (e.g., 1- methyl-2-pyrrolidinone)
Procedures for Peroxide Testing
- Identify and label all peroxide forming chemicals. Peroxide testing labels are available from Research Safety.
- Date when (1) received and (2) when opened.
- Visually inspect each container. If there is crystallization or discoloration, treat the bottle as if it contains a dangerous level of peroxides. Notify Research Safety IMMEDIATELY.
- Dispose of all peroxide formers by the expiration date on the bottle. If the expiration date is not printed, use the following rules: i. Group I- 3 months from manufactured date ii. Group II & III- 12 months from manufacture date (if inhibited); 3 months if uninhibited.
- Group I-3 months from manufacture date
- Group II & III-12 months from manfucature date (if inhibited); 3 months if uninhibited
- Test each peroxide former monthly. Record the (1) date, (2) your initials, and (3) the peroxide concentration (if any) on the peroxide testing label. If the concentration of peroxides is above 10 ppm, contact Research Safety for neutralization and disposal.
- Store peroxide formers in sealed, dark containers with a tight-fitting cap. Avoid heat and light.
Peroxide test strips are available without charge from Research Safety. Use the key below to determine the concentration of peroxides (if any):
Peroxide Table
| GROUP I MATERIALS | ||
|---|---|---|
| Diethyl Ketene | Isopropyl ether | Sodium Amide |
| Divinyl acetylene | Potassium Amide | Vinylidene Chloride |
| Divinyl Ether | Potassium Metal | |
| GROUP II MATERIALS | ||
|---|---|---|
| Acetal | Dicyclopentadiene | Methyl actylene |
| Acetaldehyde | Diethylene glycol dimethyl ether | Methyl cyclopentane |
| Cumene | Dioxane | Methyl-isobutyl ketone |
| Cyclohexene | Ethyl ether | Tetrahydrofuran |
| Cyclopentene | Ethylene glycol dimethyl ether | Tetrahydronapthalene |
| Decalin | Furan | |
| GROUP III MATERIALS | ||
|---|---|---|
| 1,3 butadiene | Chlorotrifluoroethylene | Vinyl acetate |
| Acrylic Acid | Methyl Methacrylate | Vinyl acetylene |
| Acrylonitrile | Styrene | Vinyl chloride |
| Chlorobutadiene | Tetrafluoroethylene | Vinyl pyridine |
7.3.3 Peracids and Peroxy Compounds38
Reactions and subsequent operations involving peracids and peroxy compounds should be run behind a safety shield. For relatively fast reactions, the rate of addition of the peroxy compound should be slow enough so that it reacts rapidly and no significant unreacted excess is allowed to build up. The reaction mixture should be stirred efficiently while the peroxy compound is being added, and cooling should generally be provided since many reactions of peroxy compounds are exothermic. New or unfamiliar reactions, particularly those run at elevated temperatures, should be run first on a small scale. Reaction products should never be recovered from the final reaction mixture by distillation until all residual active oxygen compounds (including unreacted peroxy compounds) have been destroyed. Decomposition of active oxygen compounds may be accomplished by the procedure described in Korach, M.; Nielsen, D. R.; Rideout, W. H. Org. Synth. 1962, 42, 50 (Org. Synth. 1973, Coll. Vol. 5, 414).
7.3.4 Polynitro Compounds
Many polynitroaromatic compounds are shock-sensitive, as are some aliphatic compounds containing more than one nitro group. Many of these compounds are sold and stored with 10 to 20 percent water, which desensitizes their reaction to shock, although they are still flammable solids. In addition to reading the SDS consult the Bretherick’s Handbook of Reactive Chemical Hazards for more in depth information on reactive hazards and associated incidents.
Storage
Polynitro compounds shall be stored separately from most chemicals and labeled so they will be easily identified as reactive. They shall not be placed in long-term storage without special posting indicating their presence and removal date. Long-term storage without checking for proper water content may allow the compounds to dehydrate sufficiently to make them highly reactive.
Surplus and waste polynitro compounds shall be given to Research Safety promptly for proper disposal or recycling and not left on a shelf to be forgotten.
If old containers of polynitro compounds are found, including picric acid or dinitrophenyl hydrazine, do not move them without consulting Research Safety. If they are moved, handle them only by the bottom of the container and never by the cap or lid, as friction may cause a violent explosion.
Picric Acid
Dry picric acid is highly explosive and should be brought into a laboratory space only when specifically required. Users should have a thorough understanding of its hazards. Although not explosive when wetted, picric acid solutions may evaporate to leave the hazardous solid. Picric acid should be stored away from combustible materials and should not be kept for extended periods. Old containers of picric acid shall be handled only by Research Safety.
Methyl nitronitrosoguanidine
Methyl nitronitrosoguanidine is a carcinogenic agent that is also shock-sensitive. It shall be stored in a separate area, preferably locked. Waste paper, plastic, and glass contaminated with this material shall be given to Research Safety for proper disposal.
7.3.5 Catalysts
Catalysts such as raney nickel or palladium on carbon shall be filtered from catalytic hydrogenation reaction mixtures with care. The catalyst has usually become saturated with hydrogen and will produce flames spontaneously on exposure to air. The filter cake should not be allowed to become dry. The funnel containing the still-moist catalyst filter cake should be put into a water bath immediately after completion of the filtration. Use a purge gas (nitrogen or argon) for hydrogenation procedures so that the catalyst can be filtered and handled under an inert atmosphere.
7.3.6 Sodium Azide
 |
Sodium azide is a toxic, highly reactive, heat-sensitive, and potentially shock-sensitive material. Because it reacts with metals, Teflon or other nonmetal spatulas shall be used. It shall be stored in a locked cabinet and used with appropriate personal protective gear.
Sodium azide should only be purchased in small quantities, ideally the minimum amount needed in the laboratory space. Consult Research Safety for a list of vendors who supply 10-gram containers of sodium azide. Storage of solid sodium azide is strongly discouraged.
Solid sodium azide, in quantities above 25 g, shall be dissolved when it arrives in the laboratory space. Solutions of sodium azide do not pose the danger of shock-sensitivity associated with the solid form; however, the hydrazoic acid generated when the azide is dissolved is extremely toxic. Therefore, the solution shall always be prepared inside a chemical fume hood.
Consult with Research Safety if planning for a reaction with greater than 5 grams of sodium azide.
7.3.7 Organometallics
Organometallics are organic compounds comprised of a metal or nonmetal attached directly to carbon (RM). Examples are Grignard compounds and metallic alkyls such as alkyl lithiums, triethylaluminum, and trimethylindium. Many organometallics are highly toxic or flammable. Many are also waterreactive and spontaneously combustible in air. Trialkyltins are the most toxic as a group. Most are highly reactive chemically. Special firefighting equipment (e.g., dry chemical powder fire extinguisher) may be needed where organometallics are handled.
7.3.8 Hydrides
Hydrides are inorganic compounds composed of hydrogen and another element, often a metal. Examples include arsine (AsH3), phosphine (PH3), diborane (B2H6), germane (GeH4), stibine (SbH3), and silane (SiH4). The listed hydrides are highly toxic and flammable. They react violently with water and oxidizing agents and pose a dangerous fire risk. Phosphine, diborane, and silane are spontaneously flammable (pyrophiric39) in air.
Certain hydride gases, notably arsine and phosphine, are commonly used as dopants in semiconductor research applications. Arsine is one of the most toxic gases known. It is a potent hemolytic agent (symptoms: red discoloration of the urine and sclera). Phosphine is extremely toxic to organs of high oxygen flow and demand. Thorough emergency planning for accidental releases shall be in place when such gases are to be used in the laboratory space. Provision of air-supply respiratory protection may be called for as well as continuous system monitoring for releases.
When waste gas streams of hydride gases are concentrated they shall be treated (e.g., scrubbing or thermal decomposition) before release into an exhaust duct. Inform Research Safety of the treatment procedures to be applied.
7.3.9 Piranha Solution, Aqua Regia and Related Etches
The preparation of these solutions requires the development of standard operating procedures. Disposal issues are addressed in the Hazardous Waste Disposal Guide.
7.4 Select Agents
Select agents/toxins are agents that the Department of Health and Human Service (HHS) considers to have the potential to pose a severe threat to human health. A list of these agents is found in the select agent regulation (42 CFR 73). A few of the Select Agents are highly toxic chemicals.
High Consequence Livestock Pathogens and Toxins are agents that the Department of Agriculture (USDA) considers to have the potential to pose a severe threat to animal or plant health, or to animal or plant products.
The plant pathogens listed by USDA have been deemed a threat to plant health or products. Agents that post a severe threat to animal health, animal products and also public health are referred to as “Overlap Agents.” These agents appear on both the HHS and USDA list of agents and toxins.
All regulatory and safety issues related to the use of select agents at NU are governed by the Institutional Biosafety Committee.
7.5 Safety Guidelines for Research with Engineered Nanomaterials
7.5.1 Definitions
Engineered nanoparticle40 means intentionally created (in contrast with natural or incidentally formed) particle with one or more dimensions greater than 1 nanomater and less than 100 nanometers. The following types of nanoparticles are beyond the scope of this definition:
- Biomolecules (proteins, nucleic acids, and carbohydrates)
- Materials for which an occupational exposure limit (OEL) or national consensus or regulatory standards exists for the nanoscale particles
- Unbound engineered nanoparticles incapable of becoming airborne or not expected to be generated or released
- Nanoscale forms of radiological materials
- Nanoparticles incidentally produced by human activities or natural processes, such as diesel engines and forest fires
Unbound engineered nanoparticle (UNP)41 means those engineered nanoparticles that, under reasonably foreseeable conditions encountered in the work, are not contained within a matrix that would be expected to prevent the nanoparticles from being separately mobile and a potential source of exposure. An engineered nanoparticle dispersed and fixed within a polymer matrix, incapable, as a practical matter, of becoming airborne, would be “bound,” while such a particle suspended as an aerosol or in a liquid would be “unbound.”
Universal Precautions42 is an approach to infection control that assumes human biological materials are potentially infectious. Under this approach, Personal Protective clothing and Equipment (PPE), work practices, and engineering controls are always used.
7.5.2 Health and Safety
Engineered very small particles exhibit properties different from larger particles of the same chemical composition, making them of interest to researchers and of potential benefit to society. Only limited information is currently available on the toxicity of engineered nanomaterials such as carbon or metal nanotubes, nanowires and sheets, fullerenes, quantum dots, carbon and metalorganic frameworks, metal or silica nanoparticles and other compounds. It is believed that exposure to some unbound engineered nanomaterials may result in health effects. Solid or liquid materials that are comprised (in part) of engineered nanomaterials may be sources of airborne nanoparticles, for example, as a result of abrasion or cutting of solid materials, drying, or agitation of liquid suspensions.
Laboratory research commonly involves handling nanomaterials in liquid suspensions or other forms that do not become easily airborne, and even UNP tend to agglomerate to a larger size. When research involves work with engineered nanoparticles for which toxicity is not yet known, it is prudent to assume the engineered nanoparticles may be toxic, and to handle the engineered nanoparticles using exposure controls that limit inhalation, skin absorption, ingestion and injection.
7.5.3 Standing Operating Procedures
See also the NIOSH Poster “Controlling Health Hazards when Working with Nanomaterials: Questions to Ask Before you Start“.
Hazard Communication
Principal Investigators purchasing, growing, or otherwise generating engineered nanomaterials in their laboratory spaces should include these materials on their chemical inventories, note their presence in the Lumen Profile and communicate required precautions and exposure controls to their laboratory members and others potentially exposed.
Engineering Controls
- Exhausted enclosures are required for handling and weighing of UNP.
- Furnaces used for engineered nanomaterials must be connected to dedicated exhaust ventilation.
- Where gram quantities of UNP are utilized in an exhausted enclosure, HEPA filtration of the exhaust air may be necessary.
Work Practices
The practices for safely working with engineered nanomaterials are essentially the same as one would use when working with any research chemical of unknown toxicity. Consideration should be taken to:
- Wet wipe work surfaces, such as benchtops where engineered nanomaterials, fibers, or solutions have been handled at the end of each day. Surface contamination with engineered nanomaterials may not be visible.
- Use gloves for handling dry or liquid materials. When nanomaterials are present in a solvent carrier, glove selection should be based on the suitability of the glove for protection against the particular solvent.
- Use universal precautions when liquid phase engineered nanomaterials are used in biological research.
- Utilize NIOSH-approved respirator filters that are rated as N-, R- or P-100 (HEPA) for respiratory protection where airborne exposure to engineered nanomaterials is expected (e.g. cleanout of systems used for growth of carbon nanotubes.).
- Dispose of engineered nanomaterials through Research Safety.
- Label engineered nanomaterials waste containers with the full chemical name or name of mixture and “engineered nanomaterials”.
- Decontaminate equipment contaminated with engineered nanomaterials before decommissioning.
Additional Sources
8.0 Compressed Gases, Cryogenic Liquids and Liquefied Gases
Hazards can result from improper handling of gas cylinders and high-pressure equipment. A leaking cylinder can produce an atmosphere that is toxic, anesthetic, asphyxiating, or explosive; and in the event of a rapid escape, a cylinder can become a randomly directed missile. The main purpose of properly handling compressed gases is, therefore, to prevent uncontrolled escape of the gas.
A non-flammable inert compressed gas is defined by the Department of Transportation (DOT) as "any material or mixture which exerts in the packaging an absolute pressure of 280 kPa (40.6 psia) or greater at 20C (68F)".
At Northwestern University most laboratory gases are ordered through Procurement.
8.1 General Precautions
- Never drop cylinders or permit them to strike each other violently.
- Do not expose cylinders to temperatures higher than 122F (50C). Some rupture disks on cylinders release at about 149F (65C).
- Never tamper with pressure relief devices in valves or cylinders.
- Before using cylinders, need all label warnings and Safety Data Sheet warnings associated with the gas being used.
- If a gas cylinder or dewar is in disrepair, affix a repair label and notify Procurement. Repair labels are available in the Research Safety offices.

8.1.1 Types of Compressed Gas Cylinders
There are three major groups of compressed gases stored in cylinders.
- Liquefied gases are partially liquid at normal temperature and charge pressure. Examples: chlorine, propane, nitrous oxide.
- Non-liquefied gases are entirely gaseous at normal temperatures regardless of charge pressure. Examples: argon, oxygen, nitrogen, hydrogen. The standard 5 foot gas cylinders supplied by gas vendors at a pressure of 2,200-2,400 psi contain an average of 250 cubic feet of gas at normal temperature.
- Dissolved gases are dissolved in a liquid phase solvent. Dissolved gas cylinders are packed with an inert, porous filter saturated with the solvent which stabilizes the volatile gas. Acetylene is the only common dissolved gas.
Some gases, such as carbon dioxide, are commonly used in both a liquid and gas form. Cylinders designed for liquid phase dispensing have a siphon, or "dip", tube.
8.1.2 Securing Cylinders
Secure the cylinder above its center of gravity (~2/3 up the cylinder). If the chain or belt is too low or too high, it will not hold the cylinder securely. Keep the cylinder valve-protection cap on when not in use. Secure cylinders by metal channels, nylon bench straps, or chains.
8.1.3 Lecture Bottles
In addition to standard precautions, the following special rules apply to work with lecture bottles in the laboratory:
- Lecture bottles do not have pressure-relief devices to prevent rupturing or a transport cap.
- Unlike larger cylinders, lecture bottles all have identical valve threads, irrespective of the gas contained within.
- If labels and valve tags do not agree, or if there is any question as to the contents of a lecture bottle, return the unused bottle to the supplier or contact Research Safety. Whenever possible, purchase lecture bottles from suppliers who will accept the return of empty or partially empty bottles.
- When transporting lecture bottles, use a cart and block the bottles to prevent rolling and falling.
8.1.4 Storage
Maximum allowable storage quantities vary depending on campus, building, floor, control area, fire rated design and type of gas. Only cylinders that are in use shall be kept in the laboratory. When the cylinder is not in use, close the main cylinder valve tightly and add the protective valve-protection cap.
Promptly remove the regulator from an empty cylinder, replace the valve-protective cap, and label the cylinder by using an “empty” tag or writing on the side of the cylinder with chalk. Never bleed cylinders completely empty; leave a slight pressure to keep contaminants out. Empty cylinders shall be promptly removed.
8.1.5 Return and Transport
Transport using a wheeled cylinder cart with the capped cylinder strapped to the cart. Do not move a gas cylinder that may have been exposed to fire or excessive heat. Notify Research Safety or Procurement.
8.1.6 Fittings and Connections
Threads on cylinder-valve outlet connections have been standardized by the Compressed Gas Association for specific gas types.

This prevents accidental mixing of incompatible gases from an interchange of connections.
Left hand fittings for fuel gases have a cut mark through the nut. Do not use cheaters or adapters that circumvent the CGA fittings.

Compression fittings require no pipe sealants. Follow the Pipe Sealants Guide.
Never lubricate, modify, force, or tamper with cylinder valves. Especially do not put oil or grease on the high-pressure side of a cylinder containing oxygen, chlorine, fluorine or another oxidizing agent. An auto ignition or explosion could result. Unlike larger cylinders, lecture bottles all have identical valve threads, irrespective of the gas contained within.
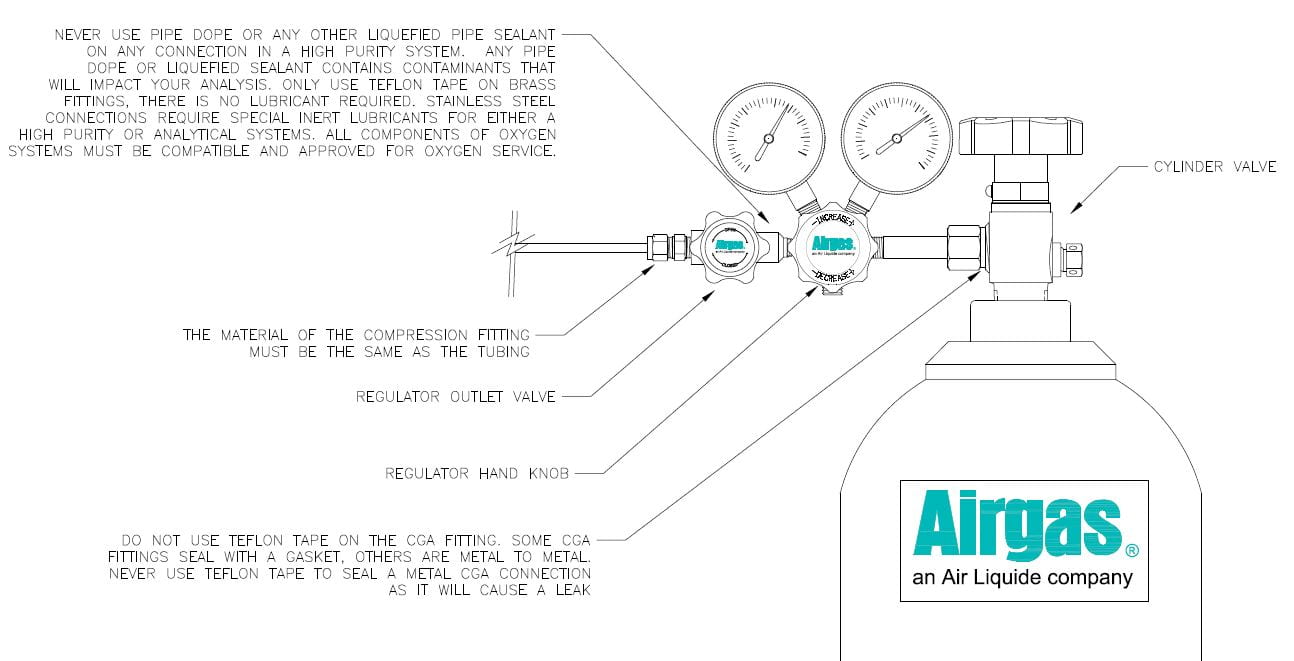
8.2 Regulators
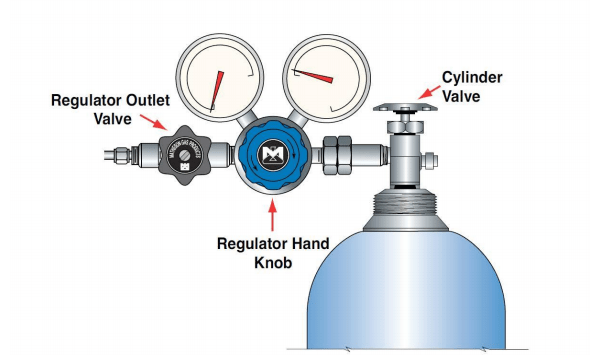
Table 8.2.1 Common Laboratory Gases and CGA Regulator Fittings
| Common Laboratory Gases | Standard CGA Fitting |
|---|---|
| Acetylene (except small cylinders) | 510 |
| Air (non-medical grade, zero air) | 590 |
| Argon | 580 |
| Carbon dioxide (fitting requires flat washer) | 320 |
| Hydrogen | 350 |
| Hydrogen chloride | 330 |
| Hydrogen sulfide | 330 |
| Methane | 350 |
| Oxygen | 540 |
| Propane | 510 |
Regulators and fittings are gas-type specific which limits interchange and adds safety. Special installation processes, not mentioned here, are used for toxic or high purity gases. Always make sure that the regulator and valve fittings are compatible.
To select the appropriate regulator:
- Determine the gas pressure needed.
- Determine the maximum pressure the system may require
- Select a delivery pressure range so the required pressures are in the 25%-90% range of the regulator delivery pressure.
- Check with the gas supplier about compatible connections and regulators.
Check cylinder outlet and regulator inlet connections for debris or contamination before connecting. Some gases, such as carbon dioxide, require a gasket/washer. Ensure that a required gasket is in place before assembling the regulator onto the cylinder.
- Tighten the connection nut with a smooth jaw wrench.
- Back out the pressure adjusting knob or key on the regulator.
- Open the cylinder valve just enough to indicate pressure on the regulator gauge (no more than one full turn).
- Check connections with a soap solution for leaks. Never use oil or grease on the regulator of a cylinder valve.
All compressed gas regulators should, at a minimum, be checked for external leakage and internal leakage (creep or crawl) regularly. Regulators should be removed from service at least every five years (more frequent in some cases) and returned to the manufacturer, or a competent agent to be inspected and/or refurbished as necessary. Regulators should also be tagged or labeled to identify the last date of inspection. Users should consult the manufacturer for specific procedures on how to check for external and internal leakage as well as the recommended frequency of the tests. Regulators are continuously exposed to high stresses due to cylinder pressures. In addition to that, the materials of construction are attacked internally by both mildly and severely corrosive gases. External corrosive environments can cause gauges and springs to corrode. Argon, helium and nitrogen regulators (CGA 580) will, under a given set of conditions, have a longer service life than regulators used for hydrogen chloride and hydrogen sulfide (CGA 330) simply because the gas service is more severe (corrosive).
The most common type of regulator failure is the internal leak, sometimes called creep or crawl. This can occur when the seat becomes damaged or displaced due to a foreign particle such as a metal chip or other material. When the seat cannot close completely, delivery pressure will not be maintained, and regulator pressure cannot reach a state of equilibrium. Downstream or delivery pressure will continue to climb until the safety relief mechanism on the regulator is activated (usually a relief valve or a diaphragm burst disk). Checking for this type of failure is relatively easy if the device has a gauge that reads regulated pressure. The gauge pressure will start to rise above the set point and continue upward. This creates a potentially hazardous condition where any downstream equipment would be subjected to pressures beyond the rated limit. Regulators should be visually checked for this type of failure. Excessive flexing of metal regulator diaphragms can cause a radial crack, which allows gas to escape to the atmosphere through the vent hole in the bonnet.
8.3 Piping and Manifolds
Piping, tubing, valves and fittings conveying hazardous materials shall be designed and installed in accordance with ASME B3143.
The system’s weakest component determines the overall pressure limit.
8.4 Toxic Gases
Purchase of diluted toxic gas, if feasible below a concentration known to be dangerous, will serve to reduce exposure risk.
Transport toxic gas cylinders with the valve-protection cap and the valve outlet cap on. Toxic-gas cylinders shall be stored in continuously mechanically ventilated enclosures with an extinguishing system44. If the net toxic gas content exceeds one pound per cylinder no more than three cylinders of toxic gas are allowed per enclosure (gas cabinet). Flow-restricting orifices are recommended on cylinders of toxic gases. All portable tanks and cylinders must be marked to indicate the orifice (inches) on the certification tags and the vessel themselves. Any new laboratory construction shall require vented gas cabinets for storage of highly toxic gases. Gas cylinder cabinets for toxic gases must have a fire extinguishing system45.
Waste toxic gases shall be treated by absorption, wet or dry scrubbing, combustion, or condensation via refrigeration, before being vented to chemical fume hoods or other local exhaust arrangements. The safe venting of pressure-relief devices should be considered46.
If the physiological warning properties for the toxic or highly toxic gas(es) are above the permissible exposure level (PEL), an emergency alarm system is required47; consult with Research Safety regarding this determination. Consult the emergency plan for the given lab area to determine the action expected during a leak situation. Contact Research Safety for information on selection, fit testing and training if respirators or Sel- Contained Breathing Apparatus (SCBA) have been provided. No one may use respirators on the job without prior medical approval, fit testing and training.
The visual alarm of a continuous leak detection system shall be blue at malfunction, yellow at the warning level and red at the danger level. A required leak detection system shall send all alarm signals to University Police.
8.5 Flammable Gases
The purchase of diluted flammable gas, if feasible below the explosive range, will serve to reduce an explosion risk.
Quantities of flammable gases allowable in Business Occupancy generally do not require installation of leak detection and emergency shutoff or excess flow control48.
Where leak detection is installed, the visual alarm of a continuous flammable gas detection system shall be yellow at the warning level and red at the danger level. A required continuous atmosphere detection system shall send all alarm signals to University Police as part of a mutually agreed upon emergency response plan.
Oxygen/fuel gas system for welding, brazing or glass blowing shall follow OSHA CFR 1910.153. This requires the use of Grade T welding hose color coded red/green for all oxy-gas welding applications and the installation of flash arresters on hydrogen and acetylene cylinders.
Flammable gas cylinders must be stored 20 feet away from oxidizers and oxygen gas cylinders or separated by a fire rated wall. When in use in an oxy/fuel system, no special separation is required.
8.5.1 Acetylene
The in-house transfer, handling, storage and utilization of acetylene in cylinders shall be in accordance with Compressed Gas Association Pamphlet G-1-2015.
Acetylene cylinders have a porous filler material filled with acetone and dissolved acetylene. The cylinder must only be used in the upright position. If a cylinder has been handled in a non-upright position, do not use it until it has sat upright for at least 30 minutes.
Do not use tubing materials, such as copper and lead solder, as they form explosive acetylides.
Never exceed the delivery pressure limit of 15psig indicated by the warning red line of an acetylene pressure gauge.
The use of an excess flow control valve is not recommended. Install a flash arrestor downstream from the regulator and check valves wherever backflow needs to be prevented.
8.5.2 Hydrogen
Individual hydrogen gas cylinders should contain less than 400 scf. Larger hydrogen gas vessels may require a laboratory designed with explosion control and other safety measures. The installation of a flash arrestor is required, and the installation of an excess flow control valve is recommended. Stainless steel (316L grade) piping and tubing is generally used for hydrogen delivery.
8.5.3 Pyrophoric Gases-Silane
Gas vessels with more than 0.5 scf (14L) of silane content above 1.37% by volume are regulated by CGA G-13. The required engineering controls for silane are much more extensive than required for flammable gases. Silane design experts should be consulted. To determine allowable quantities, binary gas mixtures containing silane concentrations between 1.37%-4.5% by volume are considered flammable. Gas mixtures with silane gas above 4.5% by volume are considered pyrophoric.
8.6 Cryogenic Liquids and Liquefied Gases
The hazards of cryogenic liquids include fire or explosion, pressure buildup, embrittlement of structural materials, asphyxiation and destruction of living tissue on contact. Liquid helium, argon or nitrogen may displace air and create an atmosphere without sufficient oxygen. Portable cylinders of cryogens must only be stored in well ventilated areas. Storage of cryogenic liquids (i.e. liquid nitrogen) or liquefied gases (i.e., carbon dioxide) in cold rooms or other rooms without external ventilation is prohibited. Fire or explosion may occur when the liquid form of flammable gases, such as hydrogen, is used without proper management of the gaseous phase. Liquid oxygen may produce an enriched oxygen atmosphere, which increases the flammability of ordinary combustible materials. Enriched oxygen levels may also cause some nonflammable materials, such as carbon steel, to burn readily.
Contact with cryogenic liquids generally causes tissue freezing and frostbite. Even brief contacts may be intense and painful. Prolonged contact may result in blood clots. Appropriate protective clothing, gloves, and eye protection — preferably a face shield — shall be worn when cryogenic liquids are handled. Choice of personal protective equipment (PPE) should be carefully evaluated, as gloves not designed for use with cryogenic liquids can saturate and cause more extensive cold damage to the skin.
8.6.1 Dewars and Transfer Equipment
Use a phase separator or special filling funnel to prevent splashing and spilling when transferring liquid nitrogen into or from a dewar. The top of the funnel should be partly covered to reduce splashing. Use only small, easily handled dewars for pouring liquid. For the larger, heavier containers, use a cryogenic liquid withdrawal device to transfer liquid from one container to another. Be sure to follow instructions supplied with the withdrawal device. The receiving vessel must be raised so the delivery tube is immediately above the mouth of the vessel (i.e., the cryogenic liquid should never be allowed to fall through air to reach the receiving vessel). When a warm tube is inserted into liquid nitrogen, liquid will spout from the bottom of the tube due to gasification and rapid expansion of liquid inside the tube. Wooden or solid metal dipsticks are recommended; avoid using plastics that may become very brittle at cryogenic temperatures.
8.6.2 Monitoring for Oxygen Deficiency
Indoor areas where bulk inert gas systems are newly installed shall be continuously monitored with an atmosphere monitoring system. The system shall provide an audible and visual alarm (red light) when the oxygen level drops to 19.5%. The audible and visual alarm shall be located inside the area and immediately outside of all entrances to the indoor area. A blue indicator light shall indicate a detection system malfunction. A required atmosphere monitoring system shall send all alarm signals to University Police.
8.7 Minimum Ventilation Rate
Natural or mechanical ventilation shall be provided when bulk inert gas systems are installed in buildings, rooms, or any indoor confined area. Ventilation shall be provided throughout the space at the rate of not less than 1.0 cubic foot per minute per square foot of floor area determined by the area enclosed. (Reference: IFC2012 – 5004.3.1)
8.8 Restricted Commodity
Investigators must order Restricted Commodity using a NUFinancials requisition. The selected requisition category must be LAB-HAZARDOUS GASES RESTRICTED. Investigators must ensure that each person placing orders, whether department office staff or laboratory staff, understands these requirements. Research Safety must review and approve all requisitions for Restricted Commodity. Research Safety will not approve purchase orders for LAB-HAZARDOUS GASES RESTRICTED intended for use outside of authorized University facilities.
8.8.1 Principal Investigator or Laboratory Supervisor Responsibilities
The Principal Investigator (PI) or laboratory supervisor shall ensure that the laboratory workers using compressed or liquefied gases and cryogenic liquids are informed of the hazards of chemicals present in their work area. Specifically, the PI or laboratory supervisor shall:
- Ensure that workers know and follow the guidelines for compressed and liquefied gases.
- Determine the required levels of protective apparel and equipment, and availability.
- Assure that the quantity of compressed and liquefied gases does not exceed Maximum Allowable Quantities (MAQs) or Restricted Commodity Thresholds of Consequence.
- Develop standard operating procedures and a hazard release risk assessment for high hazard gases.
- Maintain an up-to-date inventory record.
- Notify the business office to select the commodity category LAB-HAZARDOUS_GASES_RESTRICTED for orders of gases listed in the Appendix.
- Properly classify and record the acquisition of any Restricted Commodity within 30 days in the Research Hazards Registry Lumen.
- Ensure that facilities and training for use of any material being ordered are adequate.
- Maintain Safety Data Sheets or other medical data sheet for exposure symptoms and medical treatment for toxic gases. Inform the Emergency Medical Service providers of any unique supplies that are necessary for treatment.
The PI or laboratory supervisor shall also ensure that all laboratory workers using compressed or liquefied gases and cryogenic liquids attend mandatory Laboratory Safety training in accordance with the RS Training program. Training shall be conducted at the time of the laboratory worker’s initial assignment to a work area where compressed or liquefied gases are present and prior to assignments involving new exposure situations.
| Gaseous Chemicals | CAS# |
Concentration of Consequence in % |
Quantity Threshold of Consequence in Pounds |
|---|---|---|---|
| Arsine | 7784-42-1 | >0.6 | 15 |
| Boron trichloride | 10294-34-5 | >84.7 | 45 |
| Boron trifluoride | 7637-07-2 | >26.87 | 45 |
| Bromine chloride | 13863-41-7 | >9.67 | 45 |
| Carbonyl fluoride | 353-50-4 | >12 | 45 |
| Carbonyl sulfide | 463-58-1 | >56.67 | 500 |
| Chlorine | 7782-50-5 | >9.77 | 500 |
| Chlorine dioxide | 10049-04-4 | ACG* | any amount |
| Chlorine pentafluoride | 13637-63-3 | > 4.07 | 15 |
| Chlorine trifluoride | 7790-91-2 | >9.97 | 45 |
| Cyanogen | 460-19-5 | > 11.67 | 45 |
| Cyanogen chloride | 506-77-4 | >2.67 | 15 |
| Diborane | 19287-45-7 | >2.67 | 15 |
| Dichlorosilane | 4109-96-0 | >10.47 | 45 |
| Dinitrogen tetroxide | 10544-72-6 | >3.80 | 15 |
| Fluorine | 7782-41-4 | >6.71 | 15 |
| Germane | 7782-65-2 | >20.73 | 45 |
| Germanium tetrafluoride | 7783-58-6 | >2.11 | 15 |
| Hexafluoroacetone | 684-16-2 | >15.67 | 45 |
| Hydrogen bromide (anhydrous) | 10035-10-6 | >95.33 | 500 |
| Hydrogen chloride (anhydrous) | 7647-01-0 | ACG* | 500 |
| Hydrogen cyanide | 74-90-8 | >4.67 | 15 |
| Hydrogen fluoride (anhydrous) | 7664-39-3 | >42.53 | 45 |
| Hydrogen iodide, (anhydrous) | 10034-85-2 | >95.33 | 500 |
| Hydrogen selenide | 7783-07-5 | >0.07 | 15 |
| Hydrogen sulfide | 7783-06-4 | >23.73 | 45 |
| Methylchlorosilane | 993-00-0 | >20 | 45 |
| Methyl mercaptan | 74-93-1 | >45 | 500 |
| Nitric Oxide | 10102-43-9 | >3.83 | 15 |
| Nitrogen trioxide | 10544-73-7 | >3.83 | 15 |
| Nitrosyl chloride | 2696-92-6 | >1.17 | 15 |
| Oxygen difluoride | 7783-41-7 | >0.09 | 15 |
| Perchloryl fluoride | 7616-94-6 | >25.67 | 45 |
| Phosgene | 75-44-5 | >0.17 | 15 |
| Phosphine | 7803-51-2 | >0.67 | 15 |
| Selenium hexafluoride | 7783-79-1 | >1.67 | 15 |
| Silane49 | 7803-62-5 | >1.37 | any amount |
| Silicon tetrafluoride | 7783-61-1 | >15 | 45 |
| Stibine | 7803-52-3 | > 0.67 | 15 |
| Sulfur dioxide (anhydrous) | 7446-09-5 | >84 | 500 |
| Sulfur tetrafluoride | 7783-60-0 | > 1.33 | 15 |
| Tellurium hexafluoride | 7783-80-4 | > 0.83 | 15 |
| Trifluoroacetyl chloride | 354-32-5 | >6.93 | 45 |
| Trifluorochloroethylene | 79-38-9 | > 66.67 | 500 |
*ACG = Any commercial grade
Quantities in each building must be managed below the Threshold of Consequence or MAQ, whichever threshold is lower.
8.9 Quantity Limits on the Evanston Campus
The scope of this section generally applies to areas constructed for business occupancy (B). For quantity limits for all other occupancies, especially High Hazard Group (H) contact Research Safety.
The regulations for the Evanston Campus differ from the regulations on the Chicago Campus.
The building users on the Evanston Campus generally have to comply with the limits according to the International Building Code (IBC) and the International Fire Code (IFC). Groups of research laboratories within a building may be subdivided into fire control areas. For more specific information regarding laboratory design and fire control specification contact Facilities Management.
The total quantities of flammable or combustible liquids allowed in a fire control area (laboratory or suite of laboratories) are limited by the location in the building and the construction specifications.
| 2021 International Fire CodeMaximum Allowable Quantities in Storage per Building-Sprinklered Business Occupancy Control Areas | |||
| Hazardous Material | 1 floor below grade-2nd floor above grade | 3rd floor | 4th to 6th floor level |
|---|---|---|---|
| Cryogenic flammable | 90 gal | 67 gal | 45 gal |
| Cryogenic oxidizing | 90 gal | 67 gal | 45 gal |
|
Flammable gas (gaseous) |
2,000 cubic feeta | 1,500 cubic feeta | 1,000 cubic feeta |
|
Flammable gas (liquefied) |
150 poundsa | 112 poundsa | 75 poundsa |
|
Oxidizing gas (gaseous) |
3,000 cubic feeta | 2,250 cubic feeta | 1,500 cubic feeta |
|
Oxidizing gas (liquefied) |
150 poundsa | 112 poundsa | 75 poundsa |
|
Pyrophoric (needs approval) |
50 cubic feeta | 37 cubic feeta | 25 cubic feeta |
|
Corrosive |
1,620 cubic feeta | 1,215 cubic feeta | 810 cubic feeta |
|
Highly toxic (needs approval |
20 cubic feetb | 15 cubic feetb | 10 cubic feetb |
|
Toxic |
1,620 cubic feeta | 1,215 cubic feeta | 810 cubic feeta |
|
a. Quantities shall be increased 100% when stored in approved cabinets, gas cabinets, or exhausted enclosures as specified by the International Fire Code. b. Allowed only when stored in approved exhausted gas cabinets or exhausted enclosures. |
|||
Reference: International Fire Code (IFC) 2021 Excerpt from Tables 3806.2.1 and 5003.1.1(2)
8.9.1 Quantity Limits on the Chicago Campus
Note that the McGaw/Olson building is classified as institutional occupancy NOT business occupancy, which limits the storage quantities in accordance with NFPA 99. Contact Research Safety for further information.
Maximum Size and Quantity Limitations for Compressed or Liquefied Gas Cylinders in Laboratories Located in Buildings Classified Business Occupancy
| Flammable Gases and Oxygen | Liquefied Flammable Gases | Gases with High Health Hazard Rating | |
|---|---|---|---|
| Maximum Cylinder Size (approximate) | 10 x 50 inches | 9 x 30 inches | 4 x 15 inches |
| In sprinklered areas | 6b | 3 | 3b |
|
a. Cylinders of all toxic gases shall be kept in a continuously mechanically ventilated hood or other continuously mechanically vented enclosure, with no more than 3 cylinders per enclosure. b. In instructional laboratory work areas, the total number of cylinders shall be reduced to 3 maximumsized cylinders. Ten approximately 2″ × 12″ cylinders (lecture bottles) are allowed. In other than instructional laboratories, 25 lecture bottles are permitted. |
|||
Reference: 2019 NFPA 45
8.10 Glossary
Bulk systems
- Assembly of storage containers, pressure regulators, PRDs, vaporizers manifolds, and interconnecting piping that has a storage capacity of more than 20,000 scf (566m3) of compressed gas or cryogenic fluid including unconnected reserves regardless of storage pressure. A bulk system terminates at the point where gas at service pressure enters the supply line. (Reference: CGA P-18 2006).
Control area
- Each building’s floor has one or more control areas. This term is used in conjunction with Maximum Allowable Quantities and is further defined in the International Fire Code. A record of control area boundaries is maintained by Facilities.
Cylinder parts
 Cylinder valve-protection cap
Cylinder valve-protection cap- Valve hand wheel
- Retainer and valve stem packing nut
- Over pressure relief/safety device
- Valve outlet connection: Standardized CGA outlet (i.e., No. 350 for hydrogen service) to prevent interchange of equipment with incompatible gases
- Cylinder collar
- Valve outlet cap
- DOT identification, cylinder material, service pressure
- Cylinder serial number
- Month/Year hydrostatic test
- Other identification markings
Cylinder dimensions
 Emergency alarm system
Emergency alarm system
- A system to provide indication and warning of emergency situations involving hazardous materials.
Excess flow control
- A fail-safe system or other approved means designed to shut off flow caused by a rupture in pressurized piping systems.
Labels
- Under the Globally Harmonized System (GHS), chemical manufacturers, distributors, and importers are required to use labels that include the following: product identifier, signal word, pictogram, hazard statement, precautionary statement, and supplier information. For example:

Maximum Allowable Quantity (MAQ)
- The term is further defined in the Appendix and by the International Fire Code. A record of MAQs per control zone is maintained by Research Safety.
Oxygen deficient atmosphere
- An atmosphere in which the oxygen concentration is less than 19.5% by volume.
Permissible exposure limit (PEL)
- The allowable OSHA limit for an air contaminant in which workers may be exposed day after day without adverse health effects.
Standard cubic foot (scf)
- Cubic foot of gas measured at 70°F (21.1 °C) and 14.7 psia (101.3 kPa abs).
Restricted commodity
- Restricted in this context means that special licenses or permits are required from a federal agency before the item is allowed on campus or for a gas to exceed a threshold quantity.
Toxic gas and highly toxic gas
- A toxic gas is one with a median lethal concentration (LC50) of more than 200 and less than 2,000 parts per million by volume of gas or vapor when administered by inhalation for an hour (or less if death occurs within one hour) to albino rats weighing between 200 and 300 grams each. A highly toxic gas is characterized by a median LC50 of 200 ppm or less under the same conditions. (Reference: IFC 2012 – Chapter 2)
Sources:
Airgas Pipe Fitting Sealants, 2018
Matheson, Safe Handling of Compressed Gases in the Laboratory and Plant
Design and Safety Handbook for Specialty Gas Delivery Systems, Air Liquide America Specialty Gases
CGA P-1 – 2008 Safe Handling of Compressed Gases in Containers
CGA P-9 – 1992 The Inert Gases Argon, Nitrogen and Helium
CGA P-12 – 2009 Safe Handling of Cryogenic Liquids
CGA P-18 – 2006 Standard for Bulk Inert Gas Systems
CGA P-20 – 2009 Standard for classification of toxic gas mixtures
CGA G-1_13 – 2015 – Acetylene
CGA G-13 – 2016 Storage and Handling of Silane and Silane Mixtures
NFPA 45, Protection for Laboratories Using Chemicals, National Fire Protection Association, 1996.
International Fire Code – 2021
9.0 Chemical Waste Management
Proper handling of reaction byproducts, surplus and waste chemicals, and contaminated materials is an important part of laboratory safety procedures. Each laboratory member is responsible for ensuring that wastes are handled in a manner that minimizes personal exposure and the potential for environmental contamination.
Follow all procedures of the Hazardous Waste Disposal Guide.
10.0 Laboratory Infrastructure
10.1 Laboratory Ventilation
Laboratory spaces shall be provided with general ventilation adequate for employee comfort and sufficient to supply air for chemical fume hoods and other local ventilation devices. Because the general air supply is not adequate for manipulating hazardous materials on an open lab bench, volatile or toxic chemicals shall be handled in a chemical fume hood or other appropriate containment device.
Laboratory ventilation should change the air at least six times per hour. Higher air exchange rates may be required depending on the nature of the laboratory work. Except in special circumstances approved by Research Safety, air in laboratories shall be at a negative pressure with respect to the rest of the building. Air diffusers or grilles shall be so designed and located as to direct the air over the laboratory personnel and sweep the contaminated air away from their breathing zone. To promote uniform distribution and mixing of air in large laboratories, the supply registers shall deliver the air in all directions, at a typical velocity of 20 linear feet per minute.
Problems with general ventilation shall be reported promptly to Facilities. Adjustments or alterations to the general ventilation equipment of a laboratory space shall be performed only under the supervision of Facilities.
On occasion, Facilities will issue notices of intent to perform maintenance work on the ventilation system. These notices shall be heeded and chemical fume hoods shall not be used when Facilities is involved in repairing or adjusting the ventilation system. The supervisor of the laboratory is responsible for ensuring that the Facilities crew is informed of the hazards in the area. The chemical fume hood shall be cleared of toxic materials and properly decontaminated before such work begins. Facilities may request Research Safety to inspect the chemical fume hood prior to maintenance or repair work. Be prepared to supply a detailed history of chemical and biological agent use in the chemical fume hood for safety evaluation purposes.
10.1.2 Chemical Fume Hoods
A chemical fume hood is an important engineering control for preventing exposure to hazardous materials. In conjunction with sound laboratory techniques, a chemical fume hood serves as an effective means for capturing toxic, carcinogenic, offensive, or flammable vapors or other airborne contaminants that would otherwise enter the general laboratory atmosphere. With the sash lowered, the chemical fume hood also forms a physical barrier to protect workers from hazards such as chemical splashes or sprays, fires, and minor explosions. Chemical fume hoods may also provide effective containment for accidental spills of chemicals, although this is not their primary purpose. Due to the unknown nature of chemical vapor and gas emissions in research operations, the use of ductless hoods is prohibited. For the same reason ductless biosafety cabinets shall not be used to control hazardous chemical emissions.
Many chemical fume hood controllers are equipped with emergency purge buttons. These should be activated during an incident or if the design of an experiment fails. The button will temporarily increase total exhaust flow from the laboratory space and help remove toxic vapors or dusts from the entire space. The deliberate release and venting of chemicals (i.e., evaporation) in chemical fume hoods shall never be used as a means of disposal.
Turbulence can lead to backspill of contaminants out of the chemical fume hood. An operator has significant control over the factors that cause turbulence and, consequently, the chemical fume hood’s capture efficiency.
For example, chemical fume hoods with active experiments are not meant for storage of chemicals at the same time. Storing chemical containers and equipment in a chemical fume hood impairs its performance. The containers and equipment create turbulence as airflow is diverted around them. Volatile and odorous chemicals and highly toxic gases shall be stored in ventilated cabinets.
If chemical containers or bulky devices must be maintained in the chemical fume hood during an experiment, they should be elevated 2 to 3 inches above the interior work surface using jacks, apparatus scaffolding, support stands, ring stands, metal bars or stilts, etc. Materials remaining directly on the work surface block the incoming air and propel it back toward the chemical fume hood face. The elevation of materials in the chemical fume hood allows air to pass unimpeded to the bottom exhaust opening at the chemical fume hood’s back wall.
Turbulence is also created at the face of the chemical fume hood when obstacles to airflow such as containers and equipment are too close to the sash. Containers and equipment should always be moved 6 inches back from the inner edge of the air sill.
Even the movement of one’s hands can interrupt airflow patterns and disturb proper circulation of exhaust air. When reaching into the chemical fume hood, take care to move hands slowly with smooth gestures. If working at a chemical fume hood with a horizontal sash, use one of the panes as a barrier to splashes. Position the pane directly in front of you and move your hands on opposite sides of the pane.
Apparatus in chemical fume hoods shall be fitted with traps, condensers, or scrubbers to remove toxic fumes, gases, vapors, or dusts before venting to the atmosphere. Chemical fume hood performance is also dependent on the room’s air flow pattern, including airflow generated by drafts and persons walking by. Minimize traffic and opening and closing of doors near the chemical fume hood. When the chemical fume hood is in use, the sashes should be pulled down as far as workable for minimal external airflow interference and maximum barrier protection.
Chemical fume hoods used for hazardous chemicals shall have an average face velocity of 80 to 100 feet per minute at a minimum sash height of 12 inches. Face velocity shall not exceed 120 fpm at the working sash height.
Compounds such as perchloric acid or aqua regia are likely to cause chemical fume hood corrosion.
Chemical fume hoods shall be evaluated for performance upon installation and following any alterations. Research Safety monitors chemical fume hoods annually. The fans and duct systems are maintained and inspected by Facilities. Any problems with hood ventilation or air flow should be reported to Research Safety or Facilities for inspection and evaluation.
Refer to the Chemical Fumehood Handbook for further information regarding optimum hood operation and an understanding of ventilation principles.
10.2 Safety Showers
Safety showers shall be installed in areas where low chemical hazard operational limits are exceeded.
- Concentrated acids or bases (GHS Skin Corrosion/Irritation: Category 1): Container size limit=500ml/Total quantity limit=5 liters
- Flammable liquids: Container size limit=5 liters/Total quantity limit=20 liters
- Liquid hazardous waste: Container size limit=5 liters/Total quantity limit=20 liters
As a general rule, new shower installations shall adhere to the recommendations for shower location and minimum performance requirements established in American National Standard Z-358.1 (2009). Showers shall be placed as close to the hazard as possible, but in no case more than 10 seconds’ travel time from the hazard. Department heads shall ensure that safety showers are installed in the department locations where needed.
Every laboratory employee shall be instructed in the location(s) and use of a safety shower. Ideally, a person should be able to find the shower with his or her eyes closed. Safety showers shall provide a minimum of 20 gallons of water per minute.
Ideally, the water temperature of the shower should be tepid to prevent pain or shock to a person standing under it for 15 minutes. Safety showers shall have quick-opening valves requiring manual closing so that a person does not have to hold the valve open while trying to undress or wash off. The pull handle shall be a delta bar or large ring within easy reach but not so low as to be in the way.
Because not all laboratories have safety showers, a “Safety Shower” sign shall be placed outside each room that has a shower. Flammable-liquid cabinets or other hazardous equipment or material shall not be placed near a safety shower, and access to the shower or the activating handle shall not be impeded. The floor shall be clear in a 34-inch-diameter area under the shower.
Safety showers shall be tested and inspected at least annually. Inspection includes a visual check of visible plumbing and verification of proper operation. Facilities Management conducts the annual tests and maintains related records. Contact Facilities to schedule safety-shower testing if the shower you intend to use in an emergency has not been tested in the last 12 months.
10.3 Eyewash Fountain
An eyewash providing a continuous, low-pressure stream of aerated water shall be provided in each laboratory suite in which chemical or biological agents are used or stored and in laboratory spaces where nonhuman primates are handled. The designated eyewashes shall be labeled and easily accessible from any part of the laboratory space.
New eyewash installations shall adhere to the recommendations for minimum performance requirements established in ANSI/ISEA Z-358.1 (2009). Eyewash fountains shall supply 0.4 gallons of water per minute for 15 minutes.
Contact Research Safety for information on the standard eyewashes to be used at Northwestern University.
Bottle-type portable eyewashes are not acceptable in laboratory spaces.
PIs are responsible for ensuring that the labeled eyewash fountains in their labs are flushed weekly. Operate the valve, visually observe availability of the aerated water stream, and flush the pipes or hose of sediment that may have collected. Issue a work order to Facilities if an eyewash station does not provide a clean water stream of sufficient pressure and attach an Out-of-order sign as documentation for your maintenance action.
10.4 Laboratory Sinks and Drain Traps
Every laboratory space using chemical, radioactive or biological agents shall have at least one sink, preferably located near the room exit, available for hand washing. The sink shall be cleaned regularly to eliminate contamination, and soap shall be supplied for hand washing.
Drain traps in sinks, floors, and other places will dry out if they are not used regularly, allowing odors and contamination to back up into the room. Drain traps shall be kept filled with liquid to maintain an airlock. Also keep an airlock in drain traps of cup sinks on benches and in chemical fume hoods.
10.5 Electrical Equipment
Electrical currents of very low amperage and voltage may result in fatal shock under certain circumstances. Voltages as low as 24 volts AC can be dangerous and present a threat. Low voltage DC circuits do not normally present a hazard to human life, although severe burns are possible. The duration of contact with a live circuit affects the degree of damage, especially with regard to burns.
All electrical switches shall be labeled, including circuit breakers in the service panels, and all laboratory personnel shall know where these controls are and how to shut off circuits or equipment in case of fire or other accident. Any electrical equipment that is not operating properly or seems to be overheating shall be turned off immediately and inspected by a qualified technician.
Electrical equipment should be inspected periodically to confirm that the cords and plugs are in safe condition. Circuit diagrams, operating instructions, descriptions of hazards, and safety devices are usually provided by the manufacturer and should be kept on file for reference.
Only three-wire grounded, double insulated, or isolated wiring and equipment shall be used in 110V- 115V AC applications. All wiring and equipment shall comply with the National Electrical Code. Surge suppressor power strips in laboratory spaces shall comply with UL-1449.
In specifically designated laboratories, cold rooms, or storage rooms or other locations where concentrations of flammable vapor-air mixtures are likely to occur, certified explosion-proof wiring and equipment, including light fixtures, switches, refrigerators, and telephones, shall be used.
Series-wound motors with carbon brushes, typically found in household appliances such as blenders and mixers, are not spark-free and shall not be used in laboratory spaces where flammable vapors accumulate. Equipment manufactured for use in laboratory spaces generally contains induction motors.
Electrical extension cords should be avoided, where practical, by installing additional electrical outlets. Only electricians from Facilities are permitted to make electrical modifications in University properties. When extension cords are used, the current carrying capacity shall be larger than the current requirement of the equipment connected to it. Electrical cords on equipment shall be discarded or repaired if frayed or damaged. Cords should be kept as short as practical to avoid tripping hazards and tangles. Cable floor covers protect power cords and electrical cables from damage due to pedestrian traffic and help prevent tripping hazards. In wet locations ground fault circuit interrupters (GFCI) should be used. Power strips in laboratories must be of industrial-grade design with exception to those that solely power computers or equipment requiring special strip design or surge protection. Power strips must not be used for equipment that could require higher power loads such as furnaces, ovens, or incubators; these must be plugged directly into an appropriate electrical outlet
Place electrical equipment so as to minimize the possibility that water or chemicals could spill on it or that water could condense and enter the motor or controls. In particular, place such equipment away from safety showers. In cold rooms, condensation can be minimized by mounting electrical equipment on walls or vertical panels.
Only qualified individuals are permitted to make electrical repairs to any kind of electrical equipment. All electrical equipment shall be de-energized and tagged or locked out according to OSHA requirements before repairs are made. If adjustments or other contact are to be made with energized electrical equipment, a second person shall be present. Be sure you are not on a damp surface or touching a potential grounding surface. Use insulated tools, keep your hands dry, and wear safety glasses to prevent injury from sparks.
If a worker receives an electrical shock and is in contact with the energized device, use nonconductive gloves or a non-conducting device to pull or push the victim free from the electrical source. Help victims only if you are certain that you will not endanger your own safety. Turn off or disconnect the power source if possible. Call UP at 456. If a trained person is available, start CPR if necessary. Get medical assistance at once.
10.6 Fire Extinguisher Policy
Fire extinguishers are provided by the University in corridors, public areas, laboratory spaces, and other locations where required by building and life safety code. Facilities provides fire extinguishers in new and renovated laboratory spaces during the construction phase upon laboratory users’ requests. The standard extinguisher size for minimal and low fire hazard laboratories50 is 10 pounds – either BC or ABC. Consult with Research Safety about hazard requiring special extinguishers, e.g. metal fires.
Missing extinguishers should be reported to Facilities Management. Extinguishers in individual labs are ordered through Facilities at no cost to the PI. Call 1- 5201 to issue a work order for installation. Facilities will inspect and maintain all fire extinguishers and fire suppression systems.
Fire sprinkler heads in laboratories can be expected to release if they are exposed to temperatures above 155F. Keep high heat sources away from sprinkler heads.
11.0 Laboratory Security
11.1 Toxic Substances Control Act (TSCA)
51A laboratory security system is put in place to mitigate a number of risks and is complementary to existing laboratory security policies. In very broad terms, laboratory safety keeps people safe from chemicals, and laboratory security keeps chemicals safe from people.
There are many systems available for physical and electronic laboratory security. The choice and implementation depend on the level of security needed and resources available.
There are four integrated domains to consider when improving security of a facility:
- Physical or architectural security—doors, walls, fences, locks, barriers, controlled roof access, and cables and locks on equipment
- Electronic security—access control systems, alarm systems, password protection procedures, and video surveillance systems
- Operational security—sign-in sheets or logs, control of keys and access cards, authorization procedures, background checks, and security guards
- Information security—passwords, backup systems, shredding of sensitive information.
These domains are complementary, and each should be considered when devising security protocols.
Laboratories that possess Chemicals of Interest (COI) and are covered by the Chemical Facilities AntiTerrorism Standards (CFATS) are subject to U.S. Department of Homeland Security (DHS) requirements. The Chemical Facility Anti-Terrorism Standards are concerned with the following types of chemicals:
- EPA Risk Management Plan chemicals
- Highly toxic gases
- Chemical weapons convention chemicals
- Explosives
- Precursors of the above chemicals
The PI shall record all acquisitions of Chemicals of Interest within 30 days.
For most laboratories, there are a few general security requirements; however, most security measures are based on an assessment of the vulnerabilities and needs of an individual laboratory or institution. For some materials or operations, regulations or strict guidance documents specify the type or level of security. Special security requirements exist for regulated select agents, including toxins, and controlled substances. Radioactive materials and cyanides or other highly toxic chemicals must be secured when left unattended.
Within a laboratory, perhaps the most obvious form of physical security is the door lock. There are many choices available. Users should be trained to not hold doors open for others, and that everyone needs to use their key to pass through an access point. Unauthorized personnel should not be allowed to enter the laboratory, and if there is any question, laboratory personnel should be instructed to call University Police for guidance.
For assistance with security vulnerability assessments and security plans contact University Police.
11.2 Drug Enforcement Administration Controlled Substances and List I and List II Chemicals
In order to obtain controlled substances for research, an investigator needs both an Illinois controlled substance license and a federal controlled substance license. Contact the regulatory agencies directly for further information and applications. Properly licensed persons are permitted to possess Drug Enforcement Administration (DEA) controlled substances for research purposes. Controlled substances must be kept locked up within a laboratory space. For further information on prohibited activities see the University’s Policy on Drugs and Alcohol.
Access to gaseous anesthetics and the operation of anesthesia machines shall be limited to authorized users. Authorized users, in this context, shall have completed safety awareness training and be in compliance with the licensing requirements.
The Occupational Health and Safety Administration (OSHA) does not have a recommended exposure limit for isoflurane and defers to the American Conference of Governmental Industrial Hygienists (ACGIH) and National Institute for Occupational Safety and Health (NIOSH). ACGIH has established the isoflurane threshold limit value (TLV) as 75 ppm (566 mg/m3). NIOSH recommends that workers not be exposed to halogenated anesthetics at concentrations higher than 2 ppm. The University opts to comply with the more stringent NIOSH recommended exposure limit of 2 ppm to ensure the safety of researchers.
If monitoring of gaseous anesthetics reveals that levels are above the NIOSH recommended limits, then levels shall be either immediately reduced by a procedural change or equipment modification. The department head and PI shall comply with the requirements of the NIOSH TLV recommendations for the anesthetic. Periodic monitoring and termination of monitoring, as well as employee notification are required until all exposure levels are below the TLV limits. Medical surveillance may be a requirement as well.
If you are pregnant or planning to become pregnant, contact Research Safety so we can provide guidance in minimizing anesthetic exposure risk.
In addition, the DEA controls chemicals that can be used to manufacture controlled substances under the Domestic Chemical Diversion Control Act of 1993. Before selling DEA regulated chemicals, a supplier must verify the identity of the ordering party at Northwestern University. To accomplish this, suppliers require completion of an authorization form prior to shipment. Principal investigators and department administrators may authorize delivery of such chemicals for research purposes only. Contact Research Safety if you intend to ship such listed chemicals off campus.
12.0 Field Research
Most personal safety aspects for field research activities including training, insurance, safety advisories, trip registry, immunizations, and emergency planning checklist are addressed by Global Safety and Security.
There are restrictions about transporting hazardous materials domestically and internationally. Services for shipping of hazardous chemicals, radioactive materials and other restricted commodity off campus require some preplanning for compliance. Bringing potentially infectious research materials from field research activities onto campus also requires preplanning.
Removing restricted commodity and Class 4 lasers from campus without notification of researchsafety@northwestern.edu is prohibited.
Northwestern University employees officially sponsored to perform hazardous field research activities off campus should notify researchsafety@northwestern.edu.
The Export Compliance & International Compliance (ECIC) team assist in navigating the regulations on export controls. Heed federal export restrictions that regulate the distribution of technology, services and information.
Works Cited
1. Excerpts from Prudent Practices in the Laboratory, National Research Council 2011, pg. 267
2. Excerpts from Prudent Practices in the Laboratory, National Research Council 2011, pgs 270-272
3. OSHA Laboratory Standard
4. Excerpts from Prudent Practices in the Laboratory, National Research Council 2011 pgs. 40-41
5. Excerpts from Prudent Practices in the Laboratory, National Research Council 2011, pg. 34, 149
6. Excerpts from Prudent Practices in the Laboratory, National Research Council 2010, pgs. 137-138
7. Excerpts from Prudent Practices in the Laboratory, National Research Council 2011 pgs. 111-112
8. Excerpted from Prudent Practices in the Laboratory, National Research Council 2011, pgs. 74, 133, 140, 153, 174-175
9. Labware Chemical Resistance Table, Thermo Scientific
10. Excerpted from Prudent Practices in the Laboratory, National Research Council 2011, pg. 75, 109
11. Excerpted from Prudent Practices in the Laboratory, National Research Council 2011, pgs. 75, 76, 159
12. Excerpted from Prudent Practices in the Laboratory, National Research Council 2011, pgs. 75, 76, 159
13. American Chemical Society, “Creating Safety Cultures in Academic Institutions” (2012) and “Identifying and Evaluating Hazards in Research Laboratories” (2015)
14. Reagent Chemicals, MCB Manufacturing Chemists, Inc., 1981, pp. 359-402.
15. OSHA Laboratory Standard
16. Excerpts from Prudent Practices in the Laboratory, National Research Council 2011, pg. 97
17. NFPA 45 – Standard for Laboratories Using Chemicals – Chapter 10 Flammable and Combustible Liquids
18. Excerpts from Prudent Practices in the Laboratory, National Research Council 2011, pg. 70, 140, 191
19. 2012 International Fire Code 5003.9.8
20. Excerpted from Prudent Practices in the Laboratory, National Research Council 2011, pgs. 72, 159-161
21. Excerpt from Prudent Practices in the Laboratory, National Research Council 2011, pg. 156
22. Chemical Reactivity Assessments in R&D, David Leggett, PhD, CChem, MRSC
23. Excerpt from Prudent Practices in the Laboratory, National Research Council 2011, pg. 158
24. Excerpt from Prudent Practices in the Laboratory, National Research Council 2011, pgs. 173-174
25. Parr No.230M Safety in the Operation of Laboratory Reactors and Pressure Vessels
26. Pressure Classification of Reactions, NFPA 45 Standard on Fire Protection for Laboratories Using Chemicals (2011 Edition) Annex C 45-39
27. Excerpt from Prudent Practices in the Laboratory, National Research Council 2011, pgs. 175
28. Excerpt from Prudent Practices in the Laboratory, National Research Council 2011, pgs. 175
29. Source: The Rotary Evaporator
30. Excerpts from Prudent Practices in the Laboratory, National Research Council 2011, pgs. 172-173
31. 2012 International Fire Code 5003.9.8
32. Reference: 15-24-310 Municipal Code of Chicago – Flammable Liquids ARTICLE III. CLOSED CONTAINER STORAGE General requirements
33. Chemical Reactivity Assessments in R&D, David Leggett, PhD, CChem, MRSC
34. 2011 NFPA 45 10.3.3
35. The DOW Chemical Company, Lab Safety Academy 2014
36. Excerpts from Prudent Practices in the Laboratory, National Research Council 2011, pg. 72
37. Excerpts from Prudent Practices in the Laboratory, National Research Council 2011, pgs. 72, 100, 133
38. From note in Organic Syntheses, 2011
39. A chemical that will ignite spontaneously in air at a temperature of 130F (54.4C) or below.
40. The definition is adopted from the US Department of Energy Notice N456.1 – THE SAFE HANDLING OF UNBOUND ENGINEERED NANOPARTICLES, Approved 01/05/09
41. The definition is adopted from the US Department of Energy Notice N456.1 – THE SAFE HANDLING OF UNBOUND ENGINEERED NANOPARTICLES, Approved 01/05/09
42. The definition is adapted from the Centers for Disease Prevention and Control (CDC) universal precautions designed to prevent transmission of bloodborne pathogens in the work place.
43.IFC 2012-5003.2.2
44. IFC 2012-Section 6004.1.2
45. IFC 2012-6004.1.2
46. IFC 2012-Section 6003.1.3 Treatment Systems
47. IFC 2012-6004.2.2.10
48. IFC 2012-5003.1.4
49. Not a CFATS regulated gas
50. 2015 NFPA 45 Table 9.1.1
51. Excerpt from Prudent Practices in the Laboratory, National Research Council 2011, pgs. 256ff